
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
The shift towards being as environmentally-friendly as possible has resulted in the need for this important volume on the topic of reactions in water. Edited by one of the leaders in the field, Professor C.-J. Li, this is an essential resource for anyone wishing to gain an understanding of the world of green chemistry, as well as for chemists, environmental agencies and chemical engineers.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Green Solvents, Volume 5 by Paul T. Anastas in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Environmental Science. We have over one million books available in our catalogue for you to explore.
Information
1
The Principles of and Reasons for Using Water as a Solvent for Green Chemistry
1.1 Introduction
Chemical reactions used to manufacture important compounds such as medicinals are essentially always carried out in solution, and this is also true of the research work that is used to invent the new compounds and to develop appropriate ways to manufacture them. In the past, continuing into the present, the solvents used are normally volatile organic compounds (VOCs), and these pose an environmental problem. Their vapors can contribute to the greenhouse effect that causes global warming, and in some cases the solvent vapors can catalyze the destruction of the ozone layer that protects the Earth and its living inhabitants from short-wavelength ultraviolet solar radiation. The vapors may also be toxic to humans, plants, or animals, or they may cause diseases.
The liquids themselves can be a problem. If they are released into the earth, rivers or the ocean, they can cause direct environmental damage, while also slowly releasing their vapors. In principle, the solvents can be completely captured and purified for reuse during manufacturing, but it is difficult to prevent some loss to the environment. Hence there is interest in using environmentally benign liquids as the solvents in chemical reactions.
One possibility is supercritical carbon dioxide, which is a liquid under pressure and which has attractive solvent properties. However, unless it is completely contained and reused, it will release gaseous carbon dioxide, a greenhouse gas. Thus interest has increasingly turned to water as the solvent for chemical reactions.
Water is the solvent in which biochemical reactions are performed in Nature, and it is environmentally benign. However, it is a good solvent only for organic chemicals that have polar groups, such as alcohols and carboxylic acids. This may not be an insuperable problem. Over 20 years ago we reported that the special selectivities seen in water solution (see below) were also seen in some water suspensions, where one soluble component reacted with one that was poorly soluble [1, 2]. We pointed out that such suspensions in water could well be generally more practical ways to use water in manufacturing [2]. Recently, Sharpless and co-workers described a remarkable acceleration of a reaction in such a suspension, which they called reactions ON water [3, 4]. The large reported rate effect was seen in only one particular case, but even without a large acceleration the selectivities that we describe below could perhaps make suspensions in water a practical way for the environmentally benign properties of water to be generally useful even with insoluble reaction components.
One industry that has switched from VOCs to water is the paint industry. We are all familiar with the water-based paints that no longer emit strong solvent odors, and these have been widely adopted for painting automobiles, for instance. It is essentially impossible to capture all the solvent vapors that are released when a vehicle is spray painted, but when the solvent is water there is no problem.
Water is not simply an environmentally benign solvent; it has special properties that are essentially unique, related to what is called the “hydrophobic effect.” This is the tendency for hydrocarbons or molecules with hydrocarbon components to avoid contact with water, and to associate instead with other hydrocarbon species in water. This is what makes aqueous soap solutions dissolve grease, and it is the driving force in biology for the associations that produce cell membranes, and that cause nucleic acids to form the famous double helix. It drives the folding of proteins into their shapes in enzymes and antibodies, and it also promotes the binding of biological substrates into enzymes and antibodies [5].
As described below, the hydrophobic effect has now been used to mimic biological chemistry and to provide remarkable selectivities in the field called biomimetic chemistry. It has even been used to permit the discovery of the geometries of the transition states for some interesting reactions, information that is otherwise inaccessible. The remainder of this chapter describes examples of the use of the unique property of water to achieve not just solubility but also selectivity, but the examples will be mainly chosen from our own work. Hence it is important to refer to a number of sources in which other authors have also described their use of water and the hydrophobic effect in chemical studies.
Some of the work of our group has been presented as chapters in the books Structure and Reactivity in Aqueous Solution [6], Green Chemistry [7], and most recently Organic Reactions in Water [8]. In addition, in various review articles our work has been placed in context with that of other groups [2, 5, 9–20]. The remainder of this chapter describes the various contexts in which we have seen the special properties of water as a solvent.
1.2 Binding of Two Species Together Driven by the Hydrophobic Effect in Water
Cyclodextrins are molecules composed of glucose units linked in rings, the most common being α-cyclodextrin (six glucose units), β-cyclodextrin (seven glucose units) and γ-cyclodextrin (eight glucose units) (Scheme 1.1). The three exposed hydroxyl groups on each glucose unit make then water soluble, but they have an internal cavity that is less polar, and that will bind hydrocarbons such as aromatic rings using the hydrophobic effect in water. In later sections it is described how such cyclodextrin–substrate complexes can catalyze reactions, imitating enzymes. Here the cases where binding alone was studied are described.
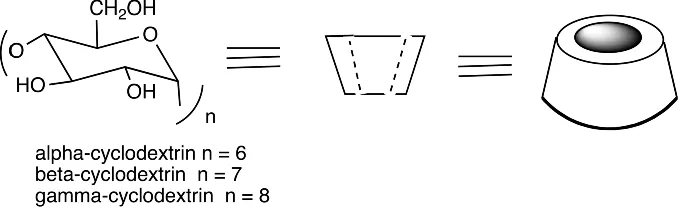
Scheme 1.1 The three cyclodextrins used – α, β, and γ-cyclodextrin – and two ways in which they are symbolized.
In one example, we saw that some dipeptides would selectively bind into simple β-cyclodextrin in water [21], and that the large steroid lithocholic acid bound strongly [22], as did cocaine [23]. When we linked two β-cyclodextrins together, we achieved even better binding of cholesterol [24], and such cyclodextrin dimers also showed strong and selective hydrophobic binding of compounds with two phenyl groups [25], of peptides with two hydrophobic amino acid components [26], and of oligopeptides whose binding promoted the formation of a helix [27].
We also tied two β-cyclodextrins with two links, which made a hinge that could let the two cyc...
Table of contents
- Cover
- Related Titles
- Title Page
- Copyright
- About the Editors
- List of Contributors
- Chapter 1: The Principles of and Reasons for Using Water as a Solvent for Green Chemistry
- Chapter 2: Green Acid Catalysis in Water
- Chapter 3: Green Bases in Water
- Chapter 4: Green Oxidation in Water
- Chapter 5: Green Reduction in Water
- Chapter 6: Coupling Reactions in Water
- Chapter 7: “On Water” for Green Chemistry
- Chapter 8: Pericyclic Reactions in Water. Towards Green Chemistry
- Chapter 9: Non-conventional Energy Sources for Green Synthesis in Water (Microwave, Ultrasound, and Photo)
- Chapter 10: Functionalization of Carbohydrates in Water
- Chapter 11: Water Under Extreme Conditions for Green Chemistry
- Chapter 12: Water as a Green Solvent for Pharmaceutical Applications
- Chapter 13: Water as a Green Solvent for Bulk Chemicals
- Index
- End User License Agreement