![]()
1
Concepts
‘Mankind is divisible into two great classes: hosts and guests.’
Max Beerbohm (b. 1872), Hosts and Guests
1.1 Definition and Development of Supramolecular Chemistry
Lehn, J.-M., ‘Supramolecular chemistry and self-assembly special feature: Toward complex matter: Supramolecular chemistry and self-organization’,
Proc. Nat. Acad. Sci. USA, 2002,
99, 4763–4768.
1.1.1 What is Supramolecular Chemistry?
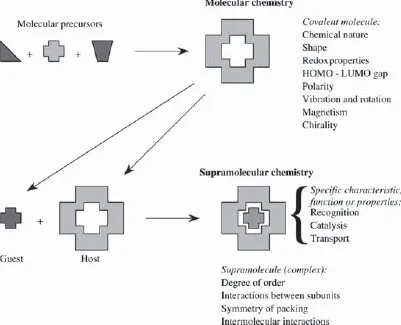
Supramolecular chemistry has been defined by one of its leading proponents, Jean-Marie Lehn, who won the Nobel Prize for his work in the area in 1987, as the ‘chemistry of molecular assemblies and of the intermolecular bond’. More colloquially this may be expressed as ‘chemistry beyond the molecule’. Other definitions include phrases such as ‘the chemistry of the non-covalent bond’ and ‘non-molecular chemistry’. Originally supramolecular chemistry was defined in terms of the non-covalent interaction between a ‘host’ and a ‘guest’ molecule as highlighted in Figure 1.1, which illustrates the relationship between molecular and supramolecular chemistry in terms of both structures and function.
These descriptions, while helpful, are by their nature noncomprehensive and there are many exceptions if such definitions are taken too literally. The problem may be linked to the definition of organometallic chemistry as ‘the chemistry of compounds with metal-to-carbon bonds’. This immediately rules out Wilkinson’s compound, RhCl(PPh3)3, for example, which is one of the most important industrial catalysts for organometallic transformations known in the field. Indeed, it is often the objectives and thought processes of the chemist undertaking the work, as much as the work itself, which determine its field. Work in modern supramolecular chemistry encompasses not just host-guest systems but also molecular devices and machines, molecular recognition, so called ‘self-processes’ such as self-assembly and self-organisation and has interfaces with the emergence of complex matter and nanochemistry (Section 1.10). The rapid expansion in supramolecular chemistry over the past 25 years has resulted in an enormous diversity of chemical systems, both designed and accidentally stumbled upon, which may lay some claim, either in concept, origin or nature, to being supramo-lecular. In particular, workers in the field of supramolecular photochemistry have chosen to adopt a rather different definition of a supramolecular compound as a group of molecular components that contribute properties that each component possesses individually to the whole assembly (covalent or non-covalent). Thus an entirely covalent molecule comprising, for example, a chromophore (light-absorbing moiety), spacer and redox centre might be thought of as supramolecular because the chromophore and redox centre are able to absorb light, or change oxidation state, whether they form part of the supermolecule or not (see Chapter 11). Similarly, much recent work has focused on the development of self-assembling synthetic pathways towards large molecules or molecular arrays. These systems often self-assemble using a variety of interactions, some of which are clearly non-covalent (e.g. hydrogen bonds) and some of which possess a significant covalent component (e.g. metal-ligand interactions, see Chapter 10). Ultimately these self-assembly reactions and the resulting self-organisation of the system rely solely on the intrinsic information contained in the structure of the molecular components and hence there is an increasing trend towards the study and manipulation of intrinsic ‘molecular information’. This shift in emphasis is nothing more than a healthy growth of the field from its roots in host-guest chemistry to encompass and inform a much broader range of concepts and activities.
Figure 1.1 Comparison between the scope of molecular and supramolecular chemistry according to Lehn.1
1.1.2 Host–Guest Chemistry
Kyba, E. P., Helgeson, R. C., Madan, K., Gokel, G. W., Tarnowski, T. L., Moore, S. S. and Cram, D. J., ‘Host-guest complexation. 1. Concept and illustration’,
J. Am. Chem. Soc., 1977,
99, 2564–2571.
If we regard supramolecular chemistry in its simplest sense as involving some kind of (non-covalent) binding or complexation event, we must immediately define what is doing the binding. In this context we generally consider a molecule (a ‘host’) binding another molecule (a ‘guest’) to produce a ‘host-guest’ complex or supermolecule. Commonly the host is a large molecule or aggregate such as an enzyme or synthetic cyclic compound possessing a sizeable, central hole or cavity. The guest may be a monatomic cation, a simple inorganic anion, an ion pair or a more sophisticated molecule such as a hormone, pheromone or neurotransmitter. More formally, the host is defined as the molecular entity possessing convergent binding sites (e.g. Lewis basic donor atoms, hydrogen bond donors etc.). The guest possesses divergent binding sites (e.g. a spherical, Lewis acidic metal cation or hydrogen bond acceptor halide anion). In turn a binding site is defined as a region of the host or guest capable of taking part in a non-covalent interaction. The host–guest relationship has been defined by Donald Cram (another Supramolecular Chemistry Nobel Laureate)2 as follows:
Complexes are composed of two or more molecules or ions held together in unique structural relationships by electrostatic forces other than those of full covalent bonds … molecular complexes are usually held together by hydrogen bonding, by ion pairing, by π-acid to π-base interactions, by metal-to-ligand binding, by van der Waals attractive forces, by solvent reorganising, and by partially made and broken covalent bonds (transition states). High structural organisation is usually produced only through multiple binding sites… A highly structured molecular complex is composed of at least one host and one guest component… A host–guest relationship involves a complementary stereoelectronic arrangement of binding sites in host and guest… The host component is defined as an organic molecule or ion whose binding sites converge in the complex… The guest component as any molecule or ion whose binding sites diverge in the complex…
This description might well be generalised to remove the word ‘organic’, since more recent work has revealed a wealth of inorganic hosts, such as zeolites (Section 9.2) and polyoxometallates (Section 9.5.2), or mixed metal-organic coordination compounds (e.g. Section 5.2), which perform similar functions and may be thought of under the same umbrella. The host–guest binding event may be likened to catching a ball in the hand. The hand, acting as the host, envelops the ball providing a physical (steric) barrier to dropping it (disassociation). This analogy falls down at the electronic level, however, since there is no real attractive force between hand and ball. Host and guest molecules and ions usually experience an attractive force between them and hence a stabilising binding free energy. The analogy does serve to introduce the term ‘inclusion chemistry’, however (the ball is included in the hand), hence the inclusion of one molecular in another.
One key division within supramolecular host–guest chemistry in its general sense relates to the stability of a host–guest complex in solution. The field of clathrate, or more generally, inclusion, chemistry, relates to hosts that are often only stable in the solid (crystalline) state and disassociate on dissolution in a solvent. Gas hydrates, urea clathrates and a wide variety of crystalline solvates (Chapter 7) fall into this category. On the other hand, molecular hosts for ions such as the crown ethers, cryptands and spherands (Chapter 3), or hosts for neutral molecules such as the carcerands and cryptophanes (Chapter 6), display significant binding both in the solid state and in solution. We should also note that there exist purely liquid-phase phenomena, such as liquid crystals and liquid clathrates, that have no direct solid-state analogies (Chapter 13).
1.1.3 Development
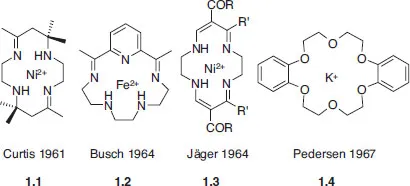
Supramolecular chemistry, as it is now defined, is a young discipline dating back to the late 1960s and early 1970s. However, its concepts and roots, and indeed many simple (and not-so-simple) supramolecular chemical systems, may be traced back almost to the beginnings of modern chemistry itself. An illustrative (although necessarily subjective and non-comprehensive) chronology is given in Table 1.1. Much of supramolecular chemistry has sprung from developments in macrocyclic chemistry in the mid-to-late 1960s, particularly the development of macrocyclic ligands for metal cations. Four systems of fundamental importance may be identified, prepared by the groups of Curtis, Busch, Jäger and Pedersen, three of which used the Schiff base condensation reaction of an aldehyde with an amine to give an imine (Section 3.10.6). Conceptually, these systems may be seen as a development of naturally occurring macrocycles (ionophores, hemes, porphyrins etc.). To these may be added the work of Donald Cram on macrocyclic cyclophanes (which dates back to the early 1950s) and, subsequently, on spherands and carcerands, and the tremendous contribution by Jean-Marie Lehn who prepared the cryptands in the late 1960s and has since gone on to shape many of the recent developments in the field.
Table 1.1 Timeline of supramolecular chemistry.
| 1810 | – | Sir Humphry Davy: discovery of chlorine hydrate |
| 1823 | – | Michael Faraday: formula of chlorine hydrate |
| 1841 | – | C. Schafhäutl: study of graphite intercalates |
| 1849 | – | F. Wöhler: β-... |