![]()
CHAPTER 1
Introduction
Ni-base alloys are one of the most important classes of engineering materials, since they can be used in a wide range of environments and applications. These alloys are selected for both aqueous and high temperature corrosion resistance, high strength at both ambient and high temperatures, ductility and toughness at low temperatures, specific electrical properties, and many other physical property-dependent applications. The Ni-base alloy welding consumables offer some properties in the as-welded condition that no other family of welding products can offer, such as the ability to be diluted by a number of diverse alloying elements while maintaining strength and ductility from cryogenic temperatures to temperatures approaching the solidus. They are also quite versatile. For example, the family of Ni-Cr-Mo welding products is used to weld 9% Ni steel to produce excellent as-welded strength and impact toughness at liquid nitrogen temperatures. Nickel and nickel-iron alloys are used to weld cast irons because they can be diluted by iron and carbon while remaining ductile and providing good machining characteristics. They are also widely used in the power generation industry for dissimilar welds between carbon steels and austenitic stainless steels in order to provide a transition in coefficient of thermal expansion for elevated temperature service.
Relative to steels, Ni-base alloys can be used both at cryogenic temperatures and temperatures approaching 1200 °C (2190 °F) because the matrix of the solid solution alloys remains austenitici from solidification to absolute zero. With appropriate alloying additions, these alloys provide useful corrosion resistance and have applications in a wide range of industries, including Power Generation, Petrochemical, Chemical Processing, Aerospace, and Pollution Control. Welding is a critical fabrication technique for Ni-base alloys. Considerable research and development has been conducted over the last 50 years in an effort to better understand and control the weldability of these alloys, and to develop welding consumables that meet the ever increasing demands on corrosion resistance and mechanical properties of welded joints.
This book is designed to provide basic information regarding the welding metallurgy and weldability of Ni-base alloys. The text includes comprehensive coverage of the two most important classes of Ni-base alloys, namely solid-solution strengthened alloys and precipitation-strengthened alloys. One chapter is dedicated to oxide dispersion strengthened (ODS) alloys and nickel aluminide alloys. In addition to providing basic information regarding the welding metallurgy of these alloy systems, concepts regarding weld repair, and selection and application of Ni-base alloys in dissimilar welds are addressed. Many important concepts are demonstrated through the use of “ case studies ” that relate these concepts to actual applications.
1.1 Ni-BASE ALLOY CLASSIFICATION
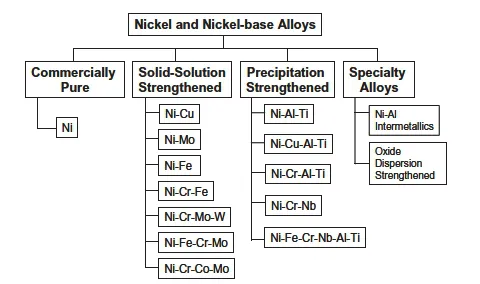
There is no systematic classification system for Ni-base alloys as there is for steels and aluminum alloys. For this reason, most Ni-base alloys are known by their trade names or by the alloy number that was originally assigned by the alloy producer. For example, INCONEL ® alloy 600ii and HASTELLOY® alloy C-22iii are also referred to as Alloy 600 and Alloy C-22. Ni-base alloys are generally classified by composition, as shown in Figure 1.1 . The following provides a brief summary of these classifications.
1.1.1 Commercially Pure Nickel Alloys
Commercially pure nickel alloys are those that contain primarily nickel (>99 wt%). There is an entire family of commercially pure nickel alloys anchored by Alloys 200 and 201. These materials have low strength and hardness, and are used principally for their corrosion resistance in caustic environments. Alloy 201 has a limit of 0.02 wt% carbon so that it can be used above 315 °C (600 °F) without the danger of being “graphitized.” Because carbon is relatively mobile in the nickel matrix above 315 °C, carbon additions beyond the solubility limit (~0.02 wt%) will result in precipitation of graphite particles that render the material brittle and weak.
There are a number of additional commercially pure nickel alloys that exist for electrical or magnetostrictive limited applications. These alloys have good weldability but are susceptible to porosity when welded. If these alloys are kept clean before and during welding, they will exhibit good resistance to cracking, but gas shielding or fluxing must be sufficient to prevent the formation of porosity. The commercially pure nickel welding consumables have additions of up to 1.5% aluminum and 2.0-to-3.5% titanium to counter the effects of even small amounts of atmospheric contamination. Both Ti and Al combine with oxygen to form oxides and nitrogen to form nitrides, thereby controlling porosity in the weld deposits
1.1.2 Solid-Solution Strengthened Alloys
Nickel and copper are isomorphous (complete solid solubility), which allows production of single phase alloys over the entire composition range. This family of materials generally exhibits good corrosion resistance to seawater and other general corrosion environments. The Ni-Cu alloys are usually quite weldable, but may be susceptible to porosity if proper shielding or welldeoxidized consumables are not used. Other solid-solution strengthened Ni-base alloys may contain only iron and most of these alloys are used for their particular coefficient of expansion or electrical properties. The Ni -36 wt% Fe alloy commonly known as INVAR ®iv exhibits the lowest coefficient of expansion of any of the Ni-base alloys and expands and contracts at a rate of less than 1.0 × 10–6 in/in/F. when heated and cooled over a range of several hundred degrees up to about 300 °F. The Ni-Fe alloys have reasonable weldability, but the development of consumables with good solidification cracking resistance with near-matching expansion properties has presented a challenge to consumable manufacturers. The Ni-Fe alloys and their consumables may also be susceptible to ductility-dip cracking. This cracking mechanism is described in detail in Chapter 3.
Other solid-solution alloys contain a variety of substitutional elements including chromium, molybdenum, and tungsten. Each element imparts particular characteristics and has the ability to alter the welding response of each alloy. Maximum ultimate tensile strength values of the solid-solution strengthened Ni-base alloys approach 120 ksi (830 MPa) with yield strengths in the range of 50 to 70 ksi (345 to 480 MPa). These alloys are used in a broad range of applications requiring good corrosion resistance. If higher strength levels are required, it is necessary to select precipitation-strengthened alloys.
1.1.3 Precipitation-Strengthened Alloys
The precipitation-strengthened Ni-base alloys contain additions of titanium, aluminum and/or niobium that form a strengthening precipitate with nickel after an appropriate heat treatment. Under most conditions, these precipitates are coherent with the austenite matrix, and thus strain the matrix such that the strength of the alloy increases substantially. The most common of these precipitates are called gamma prime [γ′-Ni3Al, Ni3 Ti, and Ni3(Ti,Al)] and gamma double prime (γ″-Ni3 Nb). By optimizing alloying additions and heat treatment, these alloys can be strengthened to reach ultimate tensile strength values exceeding 200 ksi (1380 MPa) with 0.2% offset yield strengths over 150ksi (1035 MPa).
The first precipitation-hardened Ni-Cr alloy (X-750) is strengthened by gamma prime and exhibits the combination of good oxidation resistance and high temperature strength to near the gamma prime solvus temperature. Unfortunately, it is subject to postweld strain age cracking (SAC) when welded and direct-aged without an intervening annealing treatment. This cracking mechanism is described in detail in Chapter 4. In an effort to improve weldability and avoid SAC, a second generation of precipitation-hardening Ni-Cr alloys was developed that are strengthened by gamma double prime. The most popular of these alloys is Alloy 718. Because the gamma double prime precipitate forms more slowly than gamma prime, Alloy 718 is generally immune to SAC during postweld heat treatment. One of the major applications for Alloy 718 is for aerospace gas turbine shafting and pressure containment. When melted properly to produce low levels of impurities, this alloy provides tremendous design opportunities with excellent fatigue life at service temperatures up to 760 °C (1400 °F) when properly designed.
The precipitation-strengthened alloys are often referred to as “superalloys” based on their retention of unusually high strength and corrosion resistance at elevated temperatures. The term has been loosely applied to many other high strength complex alloys, but generally the term “superalloys” is used to describe Ni-base alloys with their superior strength properties provided by the gamma prime and gamma double prime phases.
The use of “superalloys” for rotating gas turbine blades or “buckets” began with alloys such as IN713C. This alloy was similar to Alloy X-750, but was only produced as a casting and contained such high additions of aluminum and titanium that it age-hardened upon cooling from casting temperatures. Structural repairs were limited due to its extreme susceptibility to SAC, but blade tip build-up was performed without cracking. After decades of technological development, other members of the “superalloy” family were added to include the very high strength, corrosion-resistant single crystal turbine blade alloys. Welded blade tip buildup due to erosion during service is possible if the welding process is well controlled and the residual stresses are kept low. The primary challenges to weld repair of these materials include the avoidance of stray grains in the melt pool and elimination of cracking. Important processing considerations for repair of single crystal alloys are described in Chapter 6.
1.1.4 Other Specialty Alloys
There are other alloys that could fit into the “super” category by nature of impressive high temperature creep strength, such as the oxide dispersion strengthened alloys MA6000 and MA754v . These alloys exhibit superior creep strength by employing both precipitation hardening and dispersion hardening created by a fine dispersion of particles that are stable at high temperatures. Yttria (Y2O3) is one example of the dispersoid used for strengthening. These materials also have excellent high temperature oxidation resistance, but they suffer from the inability to maintain their high strength across the weld joint when joined by conventional fusion welding techniques. When melted by fusion welding, the dispersoid tends to agglomerate and the local stiffening caused by the dispersoid is lost within the fusion zone and the heat-affected zone. Welding of ODS alloys is discussed in more detail in Chapter 5 .
Nickel-aluminides are alloys designed around either the NiAl or Ni3Al compound. They exhibit very high strength and corrosion resistance, but are very difficult to weld because of their low ductility over a wide temperature range. Ni-Cr-B and Ni-Mo-Si alloys have been developed for wear resistance in a variety of environments, but these alloys are also difficult to weld due to high hardness, low ductility and the formation of low-melting range phases within their compositions.
1.2 HISTORY OF NICKEL AND Ni-BASE ALLOYS
Commercially useful Ni-base alloys were first introduced in the late nineteenth century and were developed to a high level of sophistication during the twentieth century. The element nickel was initially named by the Swedish scientist and government-sponsored mineralogist, Axel Frederik Cronstedt, in 1754 when he published “ Continuation of Results and Experiments on the Los Cobalt Ore. ” (1) . Earlier, it was known to exist as a reddish mineral, nickel arsenate-octahydrate(Ni3As2O8·8H2 O) ore that became known as Annabergite after the town of Annaberg in Saxony where it was mined. It contained 29.5% Ni and is also known as nickel bloom or nickel ochre. Miners in the area of Erzgebirge retrieved a related red-colored ore called “ nicolite ” or nickel arsenide (NiAs). Because of its reddish color, the ore was initially thought to contain copper (kupfer), but when smelted, the arsenic-bearing fumes generated were extremely noxious to the smelters and the primary metal was difficult to isolate. Thus, they thought that “ Old Nick, ” (an early reference to satan or the devil) was involved in making their work difficult and dangerous. (2) Therefore the term “ kupfer nicell ” came to be applied to the ore, which literally meant “devil’s copper.”The term Kupfer-nicell was first used in 1654 near what is present day Dresden, Germany.
It wasn’t until about 100 years later that Cronstedt officially named the element nickel after much scientific inquiry. In his words, “ Kupfernickel is the ore which contains the largest amount of the semi-metal previously described, and of which an account has been published. For this reason, I have given its regulus the same name, or, for the sake of convenience, I have called it NICKEL, until it can be proved to be only a composition of metals or semimetals previously known.”(3) Obviously, Cronstedt had only rudimentary tools for defining or analyzing the new discovery, but his pronouncement was apocryphal and the name was adopted! Over one hundred years later, a significant ore body was discovered in the Sudbury basin in Ontario, Canada and primitive mining began in the late nineteenth century. This deposit, made up principally of sulfide compounds, also contained significant amounts of copper and became an item of great interest to the Orford Copper Company in New Jersey. About the same time, nickel was being smelted and refined in Clyddich, Wales.
The International Nickel Company (INCO) was formed from the Orford Copper Company and the Canadian Copper Company on March 29, 1902. (4) In the same year, Ambrose Monell (photo in Figure 1.2) filed a patent for one of the first nickel alloys to become commercially significant. It contained approximately 2/3 nickel and 1/3 copper and is the precursor of MONEL ®vi alloy 400 (70Ni-30Cu) that is still in use today. It is not accidental that this is the ratio of nickel-to-copper found in most of the ores in the Sudbury basin. This useful alloy was produced by simply refining the ore and producing the alloy from the metallic elements found naturally.
A decade later in December 1912, another nickel producer, the Haynes Stellite company, was founded by Elwood Haynes (photo in Figure 1.3) in Kokomo, Indiana. (5) Haynes had been working on nickel and cobalt alloys with additions of chromium and was eventually granted patents on Ni-Cr and Co-Cr alloys. The Ni-Cr patent was initially rejected because of competing patents by A.L. Marsh of INCO. Haynes Stellite, after 50 years of ownership by Union Carbide Corporation and another 30 years by Cabot Corporation, is now known as Haynes International with their headquarters still located in Kokomo, Indiana. Their history is well-marked by numerous popular HASTELLOY ® alloys of the Ni-Mo, Ni-Cr-Mo and Ni-Cr-Mo-W types. Table 1.1 provides a timeline of major alloy developments over the last century.(5–7)
As exploration and mining technology improved, the supply of nickel and copper largely exceeded the demand. The onset of World War I brought about the need for ballistic steels for defense against high energy projectiles. Demand for nickel increased during the war because it was the key element that induced the hardness and toughness in these special military-oriented steels. Following World War I, the demand for nickel again languished and INCO, in a visionary moment in 1922, created the Huntington Works in Huntington, West Virginia, whose charter included the development of useful new nickel alloys and their introduction to the marketplace. The locale of Huntington, West Virginia was chosen because of three important characteristics:
1) there was an abundance of low-sulfur natural gas that would be needed for melting, annealing, and heat treating;
2) Huntington is situated near the confluence of the Guyandotte and Ohio rivers both of which were navigable; and
3) the Huntington area was populated with a ready supply of low-wage inhabitants who tilled the land and could readily be trained as mill workers.
Additionally, a rail system already served the city of Huntington, which was named for the railroad magnate Collis P. Huntington.
In 1922, INCO authorized the expenditure of $3,000,000 (approximately $40,000,000 in 2008 dollars) to build a research and development facility, and rolling mill on the east bank of the Guyandotte River in West Virginia, and the Huntington Works of INCO came into being. (8) Initially, inconsequential markets such as laundry services, and domestic and commercial kitchen sinks were fabricated using MONELL metal, as it was called initially. Later, the name was changed to MONEL ® because the U.S. Patent office would not issue a patent that contained a personal name. The alloy was chosen because of its stainless, non-rusting nature before the commercial use of stainless steels began. The facility at Huntington became the primary production facility for wrought nickel alloys and the primary location for INCO’ s nickel alloy research.
TABLE 1.1 Timeline showing the development of many Ni-base alloys.
| 1900–1909 | MONEL1 alloy 400, Ni-Cr alloys. |
| 1910–1919 | HAYNES2 alloy 6B. |
| 1920–1929 | MONEL alloy K-500, HASTELLOY A, HASTELLOY B. |
| 1930–1939 | INCONEL alloy 600, MONEL alloy R-405, PERMANICKEL alloy 300, HASTELLOY C, and HASTELLOY D |
| 1940–1949 | INCONEL alloy X-750, INCOLOY alloy 800, INCOLOY alloy 801, DURANICKEL alloy 301, HAYNES STELLITE alloy 21, HAYNES STELLITE alloy... |