
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Spectacular Chemical Experiments
About this book
Written by the author of the award-winning "Chemische Kabinettstücke" this book demonstrates over 80 enjoyable, impressive and sometimes almost unbelievable chemical experiments for the classroom, lecture hall or home. All the experiments are explained in full, and have been tested several times such that their successful reproduction is guaranteed.
Grouped into several cycles -- water, the color blue, the color red, soles, and self-organization -- the topics are perfect for experimental lectures or school projects. Detailed illustrations and the lively writing style make this book equally attractive to readers interested in chemistry, even if they are unable to perform the experiments.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part I
Water
I don’t know who discovered water, but it probably wasn’t a fish.Herbert Marshall McLuhanFrom heaven it comes,To heaven it goes,And down againTo the earthEndlessly changing.Ferdinand Fischer
| Age | Water content (%) |
| Day of birth | 79 |
| 5 years | 2 |
| 16 years | 58 |
| Adult | |
| Normal weight | 62 |
| Very slim | 69 |
| Very fat | 42 |
| Organ | Water content (%) |
| Eyeball | 99 |
| Brain | 84 |
| Heart | 74 |
| Liver | 72 |
| Bones | 55 |
| Hair | 4 |
| Teeth | 0.2 |
Experiment 1
Spontaneous Ignition by Adding Water
Whoever is ignorantof the elements,of the strength they wieldand of their qualityCannot masterThe band of the spirits.Johann Wolfgang von Goethe
Apparatus
Chemicals
Attention!
Experimental Procedure
Explanation
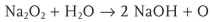
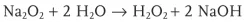

Experiment 2
Blowing-Up an Iron Ball
We ourselves are the measure of the miraculous, when we would look for a general measure, then the miraculous would disappear, and everything would have the same size.Georg Christoph Lichtenberg
Apparatus
Chemicals
Attention!
Experimental Procedure
Table of contents
- Cover
- Table of Contents
- Series Page
- Title
- The Author
- Copyright
- Foreword
- Preface
- Part I: Water
- Part II: The Color Blue
- Part III: The Color Red
- Part IV: Colloids, Sols, and Gels
- Part V: Fascinating Experiments by Self-Organization
- Part VI: Chemical Varieties
- Part VII: The Art Gallery of Chemistry
- Conclusion
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app