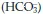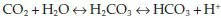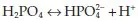![]()
Section 1
Core Issues
![]()
1
Perioperative Homeostasis
Paul Wicker
LEARNING OUTCOMES
Discuss
fluid and electrolyte balance in the perioperative patient.
Describe the
structure, function and regulation of the cardiovascular and respiratory system. Describe
blood pressure regulation.
Discuss the implications of the
metabolic response and the
stages of wound healing.
INTRODUCTION
Teamwork is the focus of good perioperative practice. Nowhere is teamwork more obvious than within the human body itself, where the close and efficient functioning of all the individual parts is essential for survival.
The purpose of this introductory chapter is to set the context of perioperative care and to identify links between the patient’s anatomy and physiology, and perioperative care. Everything that happens to the perioperative patient during surgery and anaesthesia has an effect on his or her anatomy and physiology. This makes it important to understand the internal maintenance and control of the body’s systems, and the external control through medical interventions.
The human body is a complex system of parts which can protect itself against major changes to its own internal environment. It does this by preserving a fine balance between all its major organs and systems, by maintaining fluids and electrolytes, blood pressure and oxygenation between particular limits to ensure efficient functioning. Every part of the system is related: for example, the lungs absorb oxygen which is transported by the blood to muscles such as the heart, allowing it to beat and maintain blood pressure. The blood pressure in turn pushes the oxygenated blood around the body to the tissue cells. The blood then progresses back to the lungs where it supplies the lung tissue itself, as well as going on to provide a further source of oxygenated blood to all the cells of the body.
The term homeostasis is often used to refer to the maintenance of a constant environment. In perioperative care; however, it is better to see homeostasis as a dynamic process that results in a peak state for the body under existing circumstances (Clancy et al. 2002). Hence, for example, blood pressure may or may not be maintained at preoperative levels during the peri-operative experience, and during surgery a low blood pressure may be helpful to reduce bleeding, ensuring a bloodless field. However, the main principles of control still hold true – maintaining an ideal environment for body processes to take place under current circumstances. The aim of medical interventions, as external homeostatic controllers, is to support the body’s natural ability to maintain this dynamic homeostatic environment.
The topics associated with homeostasis are huge and this chapter will highlight selected areas of interest and relate them to clinical issues raised later in the book. This chapter therefore, describes some of the ways in which the human body maintains equilibrium, how anaesthesia and surgery affect this balance and how medical interventions support the return to normal homeostasis. Finally, this chapter will look at the human body’s response to stress and the process of wound healing.
PRINCIPLES OF HOMEOSTASIS
To maintain homeostasis naturally, the body needs to:
- detect and analyse changes;
- take measures to address the changes;
- evaluate the effect of measures taken.
Control mechanisms carry out these processes, acting as receptors (detecting the changes), analysers (interpreting the changes) and effectors (acting on the changes to minimise, maximise or regulate them). These mechanisms detect changes in normal values and try to bring them back to within the normal homeostatic range. An example of this system is the acid–base balance, where the buffer systems (described later in this chapter) act in unison to maintain the pH of body fluids within a normal range of around 7.35–7.45. Under normal circumstances, the body’s buffer systems are able to maintain this balance; however, during illness, disease or because of trauma such as surgery or anaesthesia, the body may need support from external controls (medical interventions) to regain equilibrium.
Most of the body’s own control mechanisms work through negative feedback – a change occurs to the body’s environment and then mechanisms to cancel the changes are activated. Blood glucose regulation, for example, involves either the release of insulin to lower blood glucose or the release of glucagon to raise it. In either case, blood sugar levels are brought back to within the normal range.
Occasionally the control mechanisms work through positive feedback and a control mechanism to promote changes is activated – for example, blood clotting, where the blood undergoes changes that allow it to clot and therefore reduce blood loss. Once the need has passed, the blood then returns to its normal state.
Internally, the organs act as both independent and interactive homeostatic controllers. This topic goes beyond the scope of this book. However, in the perioperative patient, several systems are specifically important because they are critical to the patient’s immediate survival and are also the target of many perioperative interventions. Fluid and electrolyte balances are important perioperative considerations because of the potential for blood loss and possible hypovolaemia. Medical interventions that support the effects of blood and fluid loss, and help maintain electrolyte balance, therefore, merit consideration. The regulation of the respiratory and cardiovascular systems is also crucial in both the long and the short term. The aim of many anaesthetic functions is to control these systems to provide the optimum physiological environment during surgery.
Table 1.1 Terminology associated with fluid compartments.
| Extracellular (extracellular fluid = ECF) | Outside cells |
| Intracellular (intracellular fluid = ICF) | Inside cells |
| Interstitial | Between cells |
| Intravascular | Inside blood vessels (this is also extracellular) |
| Extravascular | Outside blood vessels (this may be intracellular, interstitial or transcellular) |
| Transcellular | Within hollow spaces, such as cerebrospinal fluid, the joints, the gastrointestinal tract, the urinary tract and the ducts of glands. Also called the ‘third space’ |
WATER AND ELECTROLYTE HOMEOSTASIS
Table 1.1 describes the fluid compartments of the body. Total body water is distributed among all the fluid compartments of the body. Of the 40 litres present in a 68kg (150 lb) male, 65% is intracellular and 35% is extracellular. Extracellular fluid is composed of 25% tissue fluid, 8% blood plasma and lymph, and 2% transcellular fluid such as cerebrospinal fluid (CSF) and synovial fluid.
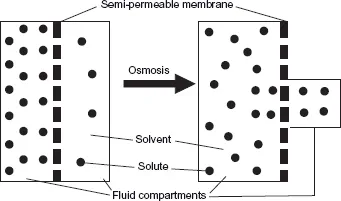
Osmosis is the main process that distributes fluid throughout these compartments (Watson & Fawcett 2003). The term ‘osmolarity’ refers to the concentration of solutes (such as potassium) in the solution (such as water). Osmosis is a special form of diffusion which involves the passage of water across a selectively permeable cell membrane that is freely permeable to water but not freely permeable to solutes. This process aims to equalise the concentrations of the solution on each side of the membrane. In osmosis, water will flow across a membrane toward the solution that has the higher concentration of solutes, because that is where the concentration of water is lowest (Figure 1.1). A solution with a high osmolarity has high osmotic pressure.
Fig. 1.1 Osmosis.
An important effect of the movement of ions and water across cell membranes is the development of an electrical charge across the membrane. The resting potential is the charge across the membrane of an undisturbed cell. When the cell is stimulated, the electrical charge may be increased, causing an ‘action potential’. In nerves, for example, this represents the movement of information away from the cell body and down the nerve. In muscles, the action potential causes the contraction of muscle fibres.
Other methods which the cell uses to transport water and electrolytes include:
- filtration, which is a passive process where hydrostatic pressure (blood pressure) forces fluid and solutes across a membrane barrier;
- carrier-mediated transport, which involves specialised cell proteins binding to ions or organic substances, facilitating their entry to or exit from the cell;
- vesicular transport, which involves the movement of materials within small membranous sacs or vesicles.
Fluid balance
A person is in a state of fluid balance when water gain equals water loss; for example, a water intake of 2.5 litres a day, taken in by food and drink, is balanced by water loss via routes such as faeces, expired air, sweat and urine.
Fluid intake is mainly controlled by the thirst centre in the hypothalamus which generates the sensation of thirst. Water output is regulated by varying urine volume. This system is mainly controlled by the antidiuretic hormone (ADH), but also to a lesser extent by aldosterone and atrial natriuretic factor (ANF). Blood osmolarity is maintained because both sodium and water are either retained or excreted.
Changes in the osmotic pressure exerted by plasma influence the release of ADH. High osmotic pressure (i.e. dehydration) leads to release of ADH; low osmotic pressure (i.e. hydration) reduces excretion of ADH. This system is so efficient that under normal conditions water balance is maintained to within 2% of the normal homeostatic range (Clancy et al. 2002).
ANF is a peptide released by walls of the cardiac atrium in response to high sodium chloride (NaCl) concentration, high extracellular fluid volume or high blood volume. ANF inhibits NaCl reabsorption in the distal convoluted tubule and cortical collecting duct of the kidneys. It also dilates the afferent glomerular arteriole and constricts the efferent glomerular arteriole. This increases the glomerular filtration rate, which increases NaCl excretion, raises urinary filtration rate and therefore increases the rate of urine production.
Volume depletion (hypovolaemia) is a loss of total body water volume when osmolarity remains normal. Vomiting, diarrhoea, burns, haemorrhage or renal failure can cause this. Addison’s disease results in dehydration leading to loss of total body water volume with an associated rise in osmolarity. This condition can also be caused by lack of drinking water, diabetes, profuse sweating or diuretics. Infants are more vulnerable to this condition.
Electrolyte balance
The balance of major electrolytes such as sodium (Na
+), potassium (K
+), calcium (Ca
2+), hydrogen (H
+) and bicarbonate
is essential to ensure homeostasis and the proper functioning of the body’s processes (Saladin 2009).
Maintaining electrolyte and water balance are three major hormones: ADH, which promotes water retention independently of Na+ and K+ concentration; aldosterone, which promotes retention of water and Na+, and secretion of K+; and ANF which increases NaCl secretion.
Electrolyte metabolism
Sodium and potassium
Sodium and potassium levels are critical to homeostasis because of their many roles. These two electrolytes contribute to maintaining membrane potentials, a major role of the sodium–potassium pump. Sodium is also responsible for 90–95% of osmolarity of extracellular fluid (ECF) and potassium is the primary cation in intracellular fluid (ICF).
The normal sodium intake of around 3–7 g/day exceeds the 0.5 g/day needed for survival. Normal blood level ranges are:
- Na+, 130–145 mmol/litre;
- K+, 3.5–5.5 mmol/litre.
The homeostasis of water, sodium and potassium levels occurs by various linked systems:
- aldosterone/ANF;
- ADH;
- oestrogen/progesterone;
- salt craving.
Intercalated cells in the collecting duct of the kidneys also control potassium levels (Saladin 2009).
Calcium
ECF contains a low concentration of calcium; however calcium exerts great influence on the body systems. Low calcium concentration increases the excitability of cells and in muscle cells this may lead to tetany. High concentrations of calcium ions make the cells less excitable and may lead to symptoms such as muscle weakness and bowel stasis. Other roles of calcium include skeletal mineralisation, muscle contraction, exocytosis (release of substances such as hormones from the vesicles of certain tissue cells) and blood clotting. Blood levels of 2.25–2.9 mmol/litre are normal.
Calcitonin is a hormone that takes part in calcium and phosphorus metabolism and affects bone deposition and resorption. Low intracellular Ca2+ levels influences calcitonin. The thyroid gland produces most calcitonin.
Phosphate
Phosphate is concentrated in ICF and variations in levels are well tolerated. Roles include being an ingredient of nucleic acids, phospholipids and some enzymes and coenzymes such as adenosine triphosphate. Phosphate also activates enzymes in metabolic pathways and buffers pH. Maintenance and control of phosphate homeostasis is by tubular reabsorption in the kidneys.
Chloride
Sodium and chloride homeostasis are linked. Chloride preserves osmolarity of ECF and plays a part in stomach acid production. The so-called ‘chloride shift’ (influx of chloride ions into cells) helps to maintain pH and electrical neutrality within cells. Chloride has a strong attraction to Na+, K+ and Ca2+, and is retained or secreted with Na+ by the kidneys.
Acid–base balance
The acid–base balance is a critical part of homeostasis – the normal pH range of ECF (including blood) is 7.35–7.45. Several processes within the body affect acid–base balance maintenance. For example, normal metabolism produces substances such as lactic acids, phosphoric acids, fatty acids, ketones and carbonic acids, which all affect pH. Even absorption of acidic foods may alter blood pH (Saladin 2009). Maintenance of acid–base balance is through buffer systems in the blood, respiration and renal systems.
Blood-based buffers
The blood itself contains three buffering systems that help to stabilise pH – the bicarbonate buffer, the phosphate buffer and the protein buffer. The bicarbonate buffering system works through the association and disassociation of carbon dioxide, water, hydrogen, carbonic acid and bicarbonate, according to the following formula:
One molecule of carbon dioxide (CO2) combines with one molecule of water (H2O) to become one molecule of carbonic acid (H2CO3). The release of hydrogen ions from the carbonic acid increases the acidity of blood.
The carbonic acid molecule is not especially stable and will break down in one of two ways. The carbonic acid molecule may break down back to carbon dioxide and water; the equation moves to the left, resulting in alkalosis. Alternatively it could break down into one molecule of bicarbonate and one hydrogen ion and the equation moves to the right, resulting in acidosis. The chemical reactions have the effect of equalising the levels of bicarbonate/hydrogen and carbon dioxide/water – so regulating the balance between alkalinity and acidity.
The phosphate buffer system works as follows:
Again, an increase in hydrogen ions i...