
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Modern Oxidation Methods
About this book
While rust is an unwanted oxidation reaction, there are also many other useful oxidation reactions that are extremely important and number among the most commonly used reactions in the chemical industry.
This completely revised, updated second edition now includes additional sections on industrial oxidation and biochemical oxidation.
Edited by one of the world leaders in the field, high-quality contributions cover every important aspect from classical to green chemistry methods:
- Recent Developments in Metal-catalyzed Dihydroxylation of Alkenes
- Transition Metal-Catalyzed Epoxidation of Alkenes
- Organocatalytic Oxidation. Ketone-Catalyzed Asymmetric Epoxidation of Alkenes and Synthetic Applications
- Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
- Modern Oxidation of Alcohols using Environmentally Benign Oxidants
- Aerobic Oxidations and Related Reactions Catalyzed by N-Hydro xyphthalimide
- Ruthenium-Catalyzed Oxidation for Organic Synthesis
- Selective Oxidation of Amines and Sulfides
- Liquid Phase Oxidation Reactions Catalyzed by Polyoxometalates
- Oxidation of Carbonyl Compounds
- Manganese-Catalyzed Oxidation with Hydrogen Peroxide
- Biooxidation with Cytochrome P450 Monooxygenases
By providing an overview of this vast topic, the book represents an unparalleled aid for organic, catalytic and biochemists working in the field.
This completely revised, updated second edition now includes additional sections on industrial oxidation and biochemical oxidation.
Edited by one of the world leaders in the field, high-quality contributions cover every important aspect from classical to green chemistry methods:
- Recent Developments in Metal-catalyzed Dihydroxylation of Alkenes
- Transition Metal-Catalyzed Epoxidation of Alkenes
- Organocatalytic Oxidation. Ketone-Catalyzed Asymmetric Epoxidation of Alkenes and Synthetic Applications
- Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
- Modern Oxidation of Alcohols using Environmentally Benign Oxidants
- Aerobic Oxidations and Related Reactions Catalyzed by N-Hydro xyphthalimide
- Ruthenium-Catalyzed Oxidation for Organic Synthesis
- Selective Oxidation of Amines and Sulfides
- Liquid Phase Oxidation Reactions Catalyzed by Polyoxometalates
- Oxidation of Carbonyl Compounds
- Manganese-Catalyzed Oxidation with Hydrogen Peroxide
- Biooxidation with Cytochrome P450 Monooxygenases
By providing an overview of this vast topic, the book represents an unparalleled aid for organic, catalytic and biochemists working in the field.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Recent Developments in Metal-catalyzed Dihydroxylation
1.1 Introduction
The oxidative functionalization of alkenes is of major importance in the chemical industry, both in organic synthesis and in the industrial production of bulk and fine chemicals [1]. Among the various oxidation products of alkenes, 1,2-diols have numerous applications. Ethylene and propylene glycols are produced annually on a multi-million tons scale as polyester monomers and anti-free ze agents [2]. A number of 1,2-diols such as 2,3-dimethyl-2,3-butanediol, 1,2-octanediol, 1,2-hexanediol, 1,2-pentanediol, and 1,2- and 2,3-butanediol are important starting materials for the fine chemical industry. In addition, enantiomerically enriched 1,2-diols are employed as intermediates in the production of pharmaceuticals and agrochemicals. Nowadays 1,2-diols are mainly manufactured by a two-step sequence consisting of epoxidation of an alkene with a hydroperoxide, a peracid, or oxygen followed by hydrolysis of the resulting epoxide [3]. Compared to the epoxidation-hydrolysis process, dihydroxylation of C=C double bonds comprises a more atom-efficient and shorter route to 1,2-diols. In general dihydroxylation of alkenes is catalyzed by osmium, ruthenium, iron, or manganese oxo species. Though considerable advances in biomimetic non-heme complexes have bee n achieved in recent years, the osmium-catalyzed variant is still the most reliable and efficient method for the synthesis of cis-1,2-diols [4]. Using osmium as a catalyst with stoichiometric amounts of a secondary oxidant, various alkenes, including mono-, di-, and tri-substituted unfunctionalized as well as many functionalized alkenes, can be converted to the corresponding diols. Electrophilic OsO4 reacts only slowly with electron-deficient alkenes; hence,itisnecessaryto employ higher amounts ofcatalyst and ligand for these alkenes. Recent studies have revealed that these substrates react much more efficiently when the reaction medium is maintained in an acidic state [5]. Citric acid appears to be superior for maintaining the pH in the desired range. However, it acts also as a ligand in this reaction but does not provide any asymmetric information transfer to the alkene. In contrast, it was found in another study that nonreactive internal alkenes, especially tetra-substituted ones, react faster at a constant pH value of 12.0 [6].
Since its discovery by Sharpless and coworkers, catalytic asymmetric dihydroxyla-tion (AD) has significantly enhanced the utility of osmium-catalyzed dihydroxylation (Scheme 1.1) [7]. Numerous applications in organic synthesis have appeared in recent years [8].
Scheme 1.1 Osmylation of alkenes.
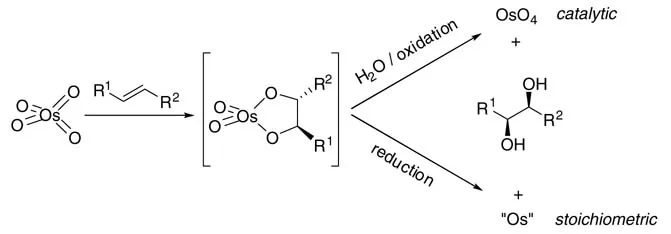
While the enantioselectivity of the reaction has largely bee n advanced through extensive synthesis and scree ning of cinchona alkaloid ligands by the Sharpless group, larger scale applications of this method remain problematic. The minimization of the useof expensive osmium catalyst and the efficient recycling of the metal should be a primary focus for the development. Cominginsecondis the replacement of the costly reoxidants, which generate overstoichiometric amounts of waste in the form of Os(VI) species.
Several reoxidation processes for osmium(VI) glycolates or other osmium(VI) species have bee n developed. Historically, chlorates [9] and hydrogen peroxide [10] were first applied as stoichiometric oxidants; however, in both cases, the dihydroxy-lation often procee ds with low chemoselectivity. Other reoxidants for osmium(VI) are tert-butyl hydroperoxide in the presence of Et4NOH [11] and a range of N-oxides such as N-methylmorpholine N-oxide (NMO) [12] (Upjohn process), and trimethyl-amine N-oxide. K3[Fe(CN)6] gave a substantial improvement in the enantioselec-tivities in asymmetric dihydroxylations when it was introduced as a reoxidant for osmium(VI) species in 1990 [13]. However, K3[Fe(CN)6] was already described as an oxidant for other Os-catalyzed oxidation reactions as early as in 1975 [14]. Today the ‘AD-mix’, a combination of the catalyst precursor K2[OsO2(OH)4], the co-oxidant K3[Fe(CN)6], the base K2CO3, and the chiral ligand, is commercially available, and the dihydroxylation reaction is easy to carry out. However, the production of over-stoichiometric amounts of waste continues to be a significant disadvantage of the reaction protocol.
This article updates an earlier version in the first edition of this book and summarizes the recent developments in the area of osmium-catalyzed dihydroxyla-tions which bring this transformation closer toa‘gree n reaction’. Special emphasis is placed on the use of new reoxidants and recycling of the osmium catalyst. Moreover, less toxic metal catalysts such as ruthenium and iron are also discussed.
1.2 Environmentally Friendly Terminal Oxidants
1.2.1 Hydrogen Peroxide
Since the publication of the Upjohn procedure in 1976, the use of N-methylmorpho-line N-oxide (NMO) based oxidants has become one of the standard methods for osmium-catalyzed dihydroxylations. However, NMO has not bee n fully appreciated in the asymmetric dihydroxylation for a long time since it was difficult to obtain high enantiomeric excess (ee ). This drawback was significantly improved by slow addition of the alkene to the aqueous tert-BuOH reaction mixture, in which 97% ee was achieved with styrene [15].
Although hydrogen peroxide was one of the first stoichiometric oxidants used in osmium-catalyzed dihydroxylation [10a], it was not employed efficiently until recently. When hydrogen peroxide is used as a reoxidant for transition metal catalysts, a very common big disadvantage is that a large excess of H2O2 is required to compensate for the major unproductive peroxide decomposition to O2.
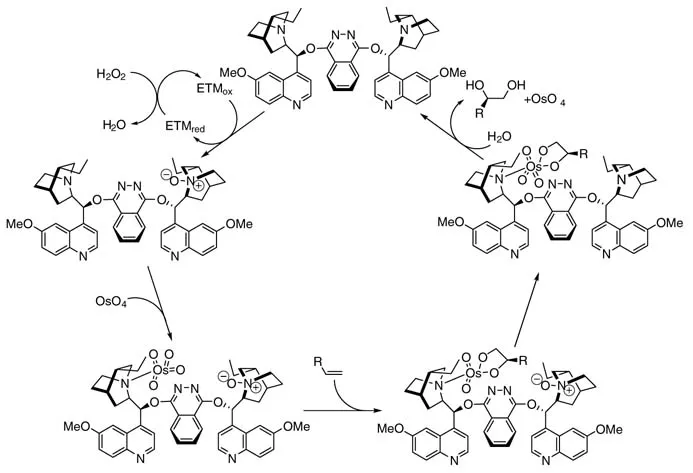
Recently, Bäckvall and coworkers were able to improve the H2O2 reoxidation process significantly by using N-methylmorpholine together with an electron transfer mediator (ETM)as co-catalysts inthe presence of hydrogen peroxide (Figure 1.1)[16]. Thus, a renaissance of both NMO and H2O2 was brought about. The mechanism of the triply catalyzed H2O2 oxidation is shown in Scheme 1.2
Scheme 1.2 Osmium-catalyzed dihydroxylation of alkenes using H2O2 as the terminal oxidant.
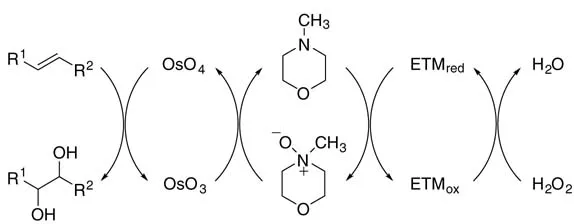
The oxidized electron transfer mediator (ETMox), namely the peroxo complexes of methyltrioxorhenium (MTO) and vanadyl acetylacetonate [VO(acac)2] and flavin hydroperoxide, generated from its reduced form (Figure 1.1) and H2O2, recycles the N-methylmorpholine (NMM)to N-methylmorpholine N-oxide (NMO), whichinturn reoxidizes the Os(VI) to Os(VIII). While the use of hydrogen peroxide as oxidant without any electron transfer mediators is inefficient and nonselective, various alkenes were oxidized to diols in good to excellent yields employing this mild triple catalytic system (Scheme 1.2)
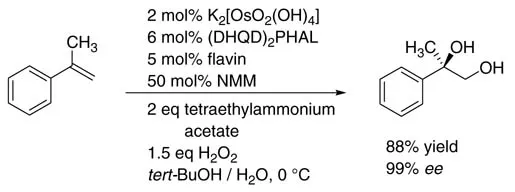
By using a chiral Sharpless ligand, high enantioselectivities were obtained. In the flavin system, an increase in the addition time for alkene and H2O2 has a positive effect on the enantioselectivity. For example, α-methylstyrene was oxidized with the aid of flavin as the ETM to its corresponding vicinal diols in good yield (88%) and excellent enantiomeric excess (99% ee ) (Scheme 1.3)
Figure 1.1 Electron transfer mediators (ETMs)
Scheme 1.3 Osmium-catalyzed dihydroxylation of a-methylstyrene using H2O2.
Bäckvall and coworkers have shown that other tertiary amines can assume the role of the N-methylmorpholine [16c]. They reported the first example of an enantiose-lective catalytic redox process where the chiral tertiary amine ligand has two different modes of operation. The primary function is to provide stereocontrol in the addition of the substrate, and the secondary function is to reoxidize the metal through the N-oxide [16c]. The results obtained with hydroquinidine 1,4-phthalazinediyl diether (DHQD)2PHAL both as electron transfer mediator and chiral ligand in the osmiumcatalyzed dihydroxylation are comparable to those obtained employing NMM together with (DHQD)2PHAL. The proposed catalytic cycle for the reaction is depicted in Scheme 1.4[16c,e].
Flavin is an efficient electron transfer mediator, but is rather unstable. Several transition metal complexes, such as vanadyl acetylacetonate, can also activate hydrogen peroxide and are capable of replacing flavin in the dihydroxylation reaction [16d,g]. The co-catalyst loading can hence be reduced from5mol% (flavin) to 2mol% [VO(acac)2 or MTO] with comparable yield and ee . The introduction of ETM for the oxidation of tertiary amine N-oxides significantly enhanced the efficiency of H2O2. With 1.5 equivalents of commercially available aqueous 30% H2O2, good to excellent yield can be achieved [16e]. Interestingly, an ETM, MTO, catalyzes oxidation of the chiral ligand to its mono-N-oxide, which in turn can be used as the oxidant to generate OsVIII from OsVI. The system gives phenylethanediol diols in 71% yield with 98% ee . This clearly confirms the role of the ETM in the regeneration of N-oxides during the dihydroxylation process.
Scheme 1.4 Catalytic cycle for the enantioselective dihydroxylation of alkenes using(DHQD)2PHAL for oxygen transfer and as source of chirality.
1.2.2 Hypochlorite
Apart from oxygen and hydrogenperoxide, bleach is the simplest and most economical oxidant, and is widely used in industry without problems. This oxidant has only bee n applied in the presence of osmium complexes in two patents in the early 1970s for the oxidation of fatty acids [17]. In 2003 the first general dihydroxylation procedure of various alkenes in the presence of sodium hypochlorite as the reoxidant was described by our group [18]. Using α-methylstyrene asa model compound, 100% conversion and 98% yield of the desired 1,2-diol were demonstrated (Scheme 1.5)
Scheme 1.5 Osmium-catalyzed dihydroxylation of a-methylstyrene using sodium hypochlorite.
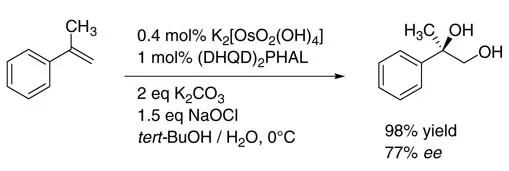
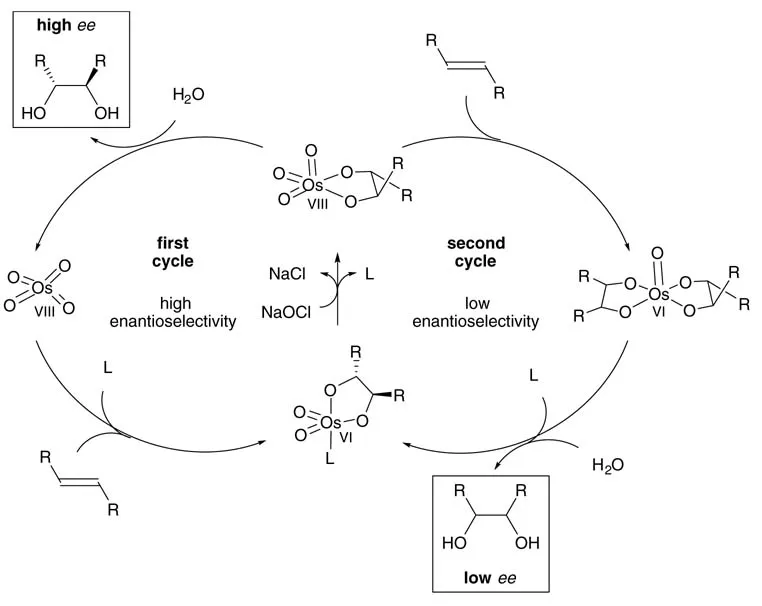
The efficacy of hypochlorite is significantly higher than that of other conventional oxidants. The yield of 2-phenyl-1,2-propanediol reached 98% after only 1h, while literature protocols using NMO [19] or K3[Fe(CN)6] [7b] gave both in 90% yield at 0°C. The turnover frequency of the hypochlorite system was 242 h-1, which is a reasonable level for further industrial application [20]. Under the conditions shown in Scheme 1.5 an enantioselectivity of only 77% ee was obtained, while 94% ee was reported using K3[Fe(CN)6] as reoxidant [7b]. The lower enantioselectivity can be explained by some involvement of the so-called second catalytic cycle with the intermediate Os(VI) glycolate being oxidized to an Os(VIII) species prior to hydrolysis (Scheme 1.6) [21].
Scheme 1.6 The two catalytic cycles in asymmetric dihydroxylation.
The enantioselectivity can be improved by applying a higher ligand concentration. In the presence of 5 mol% (DHQD)2PHAL a good enantioselectivity (91% ee ) was observed for α-methylstyrene. Using tert-butylmethylether as organic co-solvent instead of tert-butanol, 99% yield and 89% ee with only 1 mol% (DHQD)2PHAL were reported for the same substrate. An increase in the concentration of the chiral ligand in the organic phase could be an explanation of this increase in enantios-electivity. Increasing the polarity of the water phase by using a 10% aqueous NaCl solution showed a similar positive effect. Table 1.1 shows the results of the asymmetric dihydroxylation of various alkenes with NaOCl as terminal oxidant.
Table 1.1 Asymmetric dihydroxylation of different alkenes using NaOCl as the terminal oxidant.a).
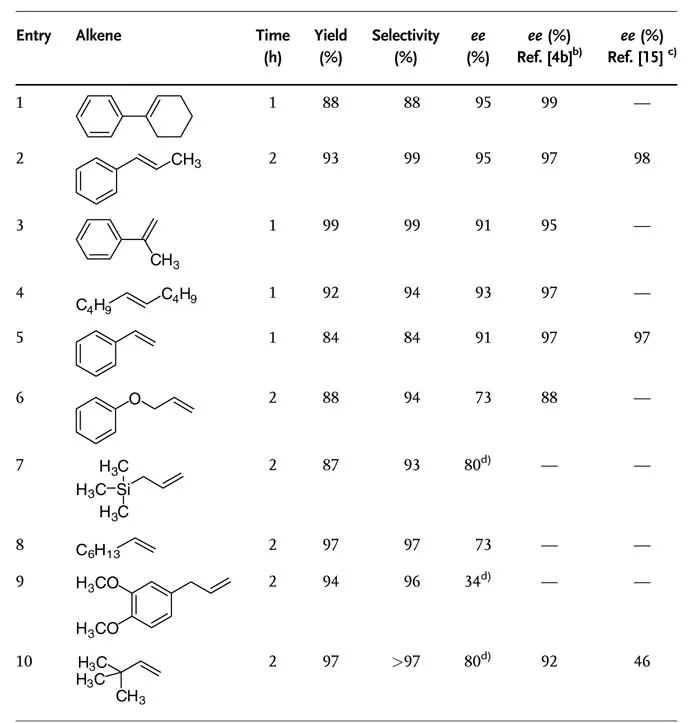
a) Reaction conditions: 2 mmol alkene, 0.4 mol% K2[OsO2(OH)4], 5 mol% (DHQD)2PHAL, 10 mL H2O, 10mL tert-BuOH, 1.5 equiv. NaOCl, 2 equiv. K2CO3, 0°C.
b) K3[Fe(CN)6] as oxidant.
c) NMO as oxidant.
d) 5 mol% (DHQD)2PYR instead of (DHQD)2PHAL.
Despite the slow hydrolysis of the sterically hindered Os(VI) glycolate, trans-5- decene reacted smoothly to give the corresponding diol. This result is especially impressive since addition of stoichiometric amounts of hydrolysis aids is usually necessary in the dihydroxylation of most internal alkenes in the presence of other oxidants.
Thus this ‘hypochlorite-procedure’ is economical, productive, and easy to manage for asymmetric dihydroxyl...
Table of contents
- Cover
- Related
- Title
- Copyright
- Table of Content
- Preface
- List of Contributors
- 1: Recent Developments in Metal-catalyzed Dihydroxylation
- 2: Transition Metal-Catalyzed Epoxidation of Alkenes
- 3: Organocatalytic Oxidation. Ketone-Catalyzed Asymmetric Epoxidation of Alkenes and Synthetic Applications
- 4: Catalytic Oxidations with Hydrogen Peroxide in Fluorinated Alcohol Solvents
- 5: Modern Oxidation of Alcohols using Environmentally Benign Oxidants
- 6: Aerobic Oxidations and Related Reactions Catalyzed by N-Hydroxyphthalimide
- 7: Ruthenium-Catalyzed Oxidation for Organic Synthesis
- 8: Selective Oxidation of Amines and Sulfides
- 9: Liquid Phase Oxidation Reactions Catalyzed by Polyoxometalates
- 10: Oxidation of Carbonyl Compounds
- 11: Manganese-Catalyzed Oxidation with Hydrogen Peroxide
- 12: Biooxidation with Cytochrome P450 Monooxygenases
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Modern Oxidation Methods by Jan-Erling Bäckvall in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.