- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Temperature-Programmed Gas Chromatography
About this book
This book provides a comprehensive up-to-date overview of temperature-programmed gas chromatography (GC). The first part of the book introduces the reader to the basics concepts of GC, as well as the key properties of GC columns. The second part describes the mathematical and physical background of GC. In the third part, different aspects in the formation of a chromatogram are discussed, including retention times, peak spacing and peak widths.
An invaluable reference for any chromatographer and analytical chemist, it provides all the answers to questions like:
- At what temperature does a solute elute in a temperature-programmed analysis?
- What is the value of the retention factor of eluting solute?
- How wide are the peaks?
- How large is the time distance between two peaks?
- How do all these parameters depend on the heating rate?
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part One
Introduction
Chapter 1
Basic Concepts and Terms
Chromatography is a technique of separation of compounds – components of a mixture. Chromatography can be analytical [1], or – as in the case of preparative chromatography [2–4] – it can be other than analytical. The purpose of analytical chromatography is to obtain information regarding a mixture and its components rather than to make a product. Here the analytical chromatography has been considered only. How clean is the air in this room? What are the major components of the gasoline in this container? Is this pesticide present in the soil at this location, and, if yes, how much of it is there? To answer these and similar questions, one can analyze a representative sample of a mixture in question – a test mixture.
A chromatograph or a chromatographic instrument consists of several devices. The key device is the separation device – the one where the separation takes place. In column chromatography, the separation device is a chromatographic column – a tube that either has a special material along its inner walls (an open tubular column) or is packed with small particles (packed column) or with a porous material. The separation occurs due to different levels of nondestructive interactions of different components (analytes, species) of a test mixture with the material inside the column. As the subject of this book is the column analytical chromatography, from now on, the term chromatography will always infer that technique.
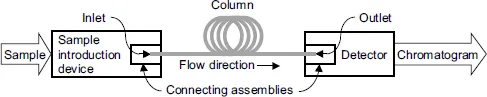
In addition to a column, a typical chromatograph includes, Figure 1.1, a sample introduction device [5–10] and a detector [5,6,10,11]. A chromatograph also requires a fluid (eluent [6, 12], mobile phase) that flows through the column from its inlet to the outlet transporting components of a test mixture in the same direction. In gas chromatography (GC), the mobile phase is an inert carrier gas, in liquid chromatography (LC), the mobile phase is a liquid. To emphasize the fact that the components of a test mixture are soluble in the mobile phase, they are also called the solutes.
Figure 1.1 Block-diagram of a chromatograph.
A set of conditions for the execution of a chromatographic analysis (a run) – the column type and temperature, the carrier gas type and its flow rate or pressure, the sample introduction device and the detector together with their operational conditions, and so forth – comprise a method of the analysis.
A chromatographic analysis starts with a quick (ideally, instantaneous) injection of a test mixture into the column inlet. While being transported through the column, different solutes differently interacting with the column interior migrate through the column with different velocities. As a result, each solute is retained (resides) in the column for different amount of time, known as the retention time or the residence time. Different retention times cause the solutes to elute – to pass through the column outlet – separately from each other constituting the separation of the solutes.
When an eluite [6, 13] (a solute eluting from a column) mixed with effluent [12] (mobile phase leaving a column) passes through a detector, the latter generates a response indicative of the presence of the solute in the detector. Ideally, a detector response to each solute should be proportional to the solute amount or concentration.
A way to observe the separation result is through a chromatogram representing a plot of a detector signal – the detector response as a function of time elapsed since the injection of a test mixture. A simple chromatogram resulted from analysis of a two-component mixture is shown in Figure 1.2. Ideally, it should be a line chromatogram [14–16] shown in Figure 1.2a. Unfortunately, no matter how quick was the injection, each solute migrating along the column occupies a zone (a band) whose width gradually increases with time. As a result, each eluite and a corresponding peak have nonzero width as shown in Figure 1.2b.
Figure 1.2 Chromatograms of a two-component mixture. The markers, tR1 and tR2, are the retention times of respective components. (a) A line chromatogram that would occur if there were no broadening of the solute zones during the solute migration along the column. (b) A realistic two-peak chromatogram resulted from the separation and the broadening of the solute zones.
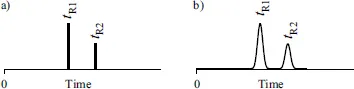
Usually, a chromatographic analysis does not end with the generation of a chromatogram. A contemporary chromatographic system might include a chromatograph and a data analysis subsystem. The latter might quantify and identify the peaks and report retention time, width, area, height, amount, concentration, and other information regarding each peak.
Two different concepts – a solute zone and a peak – have already been mentioned in the preceding text. A zone, Figure 1.3, is a space occupied by a solute migrating in a column. The distribution of a solute zone along a column can be described (Figure 1.3b) by the solute’s specific amount, a(z), – the solute amount (mass, mole, and so forth) per unit of length. The width of a zone is measured in units of length along the column. A peak, on the other hand, can be a zone elution rate [06], a detector signal in response to elution of the zone, or a portion of chromatogram (Figure 1.2b) representing that signal. In either case, the width of a peak is measured in units of time. The distinction between the terms a zone and a peak is recognized throughout the book. Typically, both a zone and a peak representing the zone have similar pulse-like shape with a clearly identifiable maximum.
Figure 1.3 Solute zone within a L-long column (a), and the distribution, a(z), of its amount along the z-axis (b). The proportions of the column and the zone shown here are not typical. Typically, a column is several orders of magnitude longer than its internal diameter, ...
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Dedication
- Preface
- Constants, Abbreviations, Symbols
- Part One: Introduction
- Part Two: Background
- Part Three: Formation of Chromatogram
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Temperature-Programmed Gas Chromatography by Leonid M. Blumberg in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.