
eBook - ePub
Proton Exchange Membrane Fuel Cells Modeling
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Proton Exchange Membrane Fuel Cells Modeling
About this book
The fuel cell is a potential candidate for energy storage and conversion in our future energy mix. It is able to directly convert the chemical energy stored in fuel (e.g. hydrogen) into electricity, without undergoing different intermediary conversion steps. In the field of mobile and stationary applications, it is considered to be one of the future energy solutions.
Among the different fuel cell types, the proton exchange membrane (PEM) fuel cell has shown great potential in mobile applications, due to its low operating temperature, solid-state electrolyte and compactness.
This book presents a detailed state of art of PEM fuel cell modeling, with very detailed physical phenomena equations in different physical domains. Examples and a fully coupled multi-physical 1.2 kW PEMFC model are given help the reader better understand how to use the equations.
Among the different fuel cell types, the proton exchange membrane (PEM) fuel cell has shown great potential in mobile applications, due to its low operating temperature, solid-state electrolyte and compactness.
This book presents a detailed state of art of PEM fuel cell modeling, with very detailed physical phenomena equations in different physical domains. Examples and a fully coupled multi-physical 1.2 kW PEMFC model are given help the reader better understand how to use the equations.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
PART 1
State of the Art: Of Fuel Cells Modeling
Chapter 1
General Introduction
1.1. What is a fuel cell?
The operating principle of the fuel cell is quite old. This principle was introduced by two researchers (Christian Friedrich Schönberg and William Grove) within a span of one month in 1839. The principle is based on the reaction between two gases: hydrogen (or a hydrogen-rich gas) used as fuel and oxygen used as oxidant. The operating principle of the fuel cell is relatively straightforward: it can be described as inverse electrolysis. More precisely, it consists of a controlled electrochemical combustion between oxygen and hydrogen resulting in the simultaneous production of electricity, water, and heat, following the global formula:
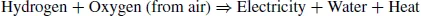
This electrochemical reaction takes place in a system which consists of two electrodes (cathode and anode) separated by an electrolyte. Depending on the type of the fuel cell, the reaction can take place at different temperatures, from a few dozens of degrees Celsius for proton exchange membrane fuel cells (PEMFC) to almost one thousand degrees Celsius for solid-oxide fuel cells (SOFC).
Although this operating principle is valid for all types of fuel cells, differences in electrolytes and operating temperatures result in different characteristics for different types of fuel cells, so that they are more or less adapted for certain applications.
For example, the operating principle of proton exchange membrane fuel cells is shown in Figure 1.1:
Figure 1.1. Operating principle of the proton exchange membrane fuel cell (PEMFC or PEFC) [BLU 09]
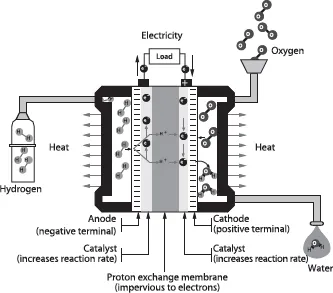
– the fuel cell is fed by hydrogen at the anode (negative terminal) and oxygen at the cathode (positive terminal);
– hydrogen molecules are dissociated through a platinum-based catalytic reaction and each hydrogen atom loses its only electron. Since the electron cannot pass through the membrane (insulator), it passes through the electrical circuit and creates an electrical current (Figure 1.1). Without its electron, the hydrogen ion (now H+, a proton) can pass through the membrane to the cathode;
– electrons coming from the anode through the electrical circuit, protons migrating from the anode through the membrane and oxygen molecules (O2) combine at the cathode to form water molecules containing two hydrogen atoms and one oxygen atom (H2O).
Water and electricity are thus produced. As the reaction is not perfect, it also produces heat that can be harnessed for various purposes (e.g. heating).
The fuel cell is an energy converter, and not an energy source. It converts the chemical energy of a fuel (hydrogen) directly into electricity and heat.
The conversion process from hydrogen to electricity is non-polluting, as the only byproduct is water. The fuel, hydrogen, is an energy carrier: it carries energy. Hydrogen is not a source of energy because it requires energy to be produced (hydrogen exists only in small quantities in nature). Hydrogen can be extracted from a primary energy source (gasoline, methane, ethanol, etc.) or produced from electrolysis of water (which is the separation of the water molecule into hydrogen and oxygen) [BLU 09].
1.2. Types of fuel cells
Various fuel cell technologies exist, where each technology has its specific advantages and drawbacks. These advantages and drawbacks render them more or less suitable for certain applications. For example, low-temperature fuel cells such as PEMFC or AFC (see section 1.2.1), start up faster than the high-temperature fuel cells, which makes them more suitable than other fuel cell technologies for transportation applications. However, these low-temperature fuel cells require larger quantities of catalyst and more bulky heat exchangers, due to the small temperature difference between the fuel cell and environment: these constraints make these fuel cells less suitable for transportation applications, in which space constraint is a key issue. For high-temperature fuel cells, the opposite is the case: they have a relatively long start-up time but require less space due to smaller heat exchangers.
The choice of the type of a fuel cell for any given application is therefore always a compromise between its inherent advantages and drawbacks. In order to overcome certain drawbacks, researchers tend to look to:
1) increase the operating temperature of low-temperature fuel cells (to approximately 120°C) for transportation applications, so as to decrease the size of the fuel cell and improve water management;
2) lower the operating temperature of high-temperature fuel cells, thereby reducing thermal constraints, start-up time and costs, while extending the lifetime of the fuel cell. An example of such an improved cell is the IT-SOFC (Intermediate-Temperature Solid Oxide Fuel Cell).
Operating temperature of the optimal fuel cell seems to be around 150–200°C [MEN 08], which corresponds to the temperature of which, in turn, has other drawbacks unfortunately.
Table 1.1. Summary of different fuel cell technologies
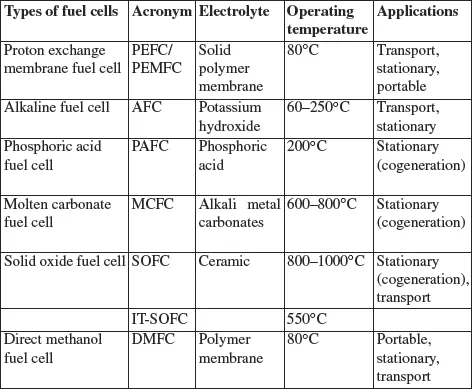
Fuel cell classification is generally based on the type of electrolyte, since the electrolyte determines the operating temperature of the cell and the type of ion which will ensure ionic conduction. The most commonly used technologies are as follows (Table 1.1):
– polymer electrolyte fuel cells (PEFC) or proton exchange membrane fuel cells (PEMFC), operating at around 80°C;
– alkaline fuel cells (AFC), operating at around 100°C;
– phosphoric acid fuel cells (PAFC), operating at around 200°C;
– molten carbonate fuel cells (MCFC), operating at around 700°C;
– solid oxide fuel cells (SOFC), operating at around 800–1000°C.
1.2.1. Proton exchange membrane fuel cell (PEMFC, PEFC)
Proton exchange membrane fuel cells operate at temperatures under 100°C, with a stack efficiency of the order of 50%. Its low-operating temperature enables these fuel cells to start up relatively quickly, making this technology particularly well adapted to transportation applications. The typical PEMFC power range is from a few milliwatts to a few hundred kilowatts.
The electrolyte of PEMFC is generally a perfluorinated polymer membrane capable of carrying hydrogen ions (i.e. protons).
The primary advantages of PEMFC are as follows:
– the electrolyte is solid: there is no risk of electrolyte leakage;
– the operating temperature is low, which means that the cell does not need a long time to warm up before being fully operational;
– the specific power is high, and can be as high as 1 kW/kg;
However, they have their own drawbacks:
– the membranes must be kept in a good degree of hydration in order to transfer hydrogen protons. If this condition is not met, there is a risk of membrane deterioration, which would lead to the degradation of the fuel cell itself;
– the necessity of platinum makes the fuel cells susceptible to contamination from carbon monoxide (CO), which poisons catalytic sites.
Besides problems associated with fabrication of the fuel cell stack, certain technical problem have to be overcome:
1) manufacturing cells capable of cold-starting in low-temperature conditions, particularly under 0°C;
2) heat and water management;
3) durability, especially in the conditions commonly found in transportation (thermal and electrical cycling, vibrations, climactic conditions, etc.).
1.2.2. Alkaline fuel cells (AFC)
During the 1960s, the Apollo missions relied on alkaline fuel cells to generate water and electricity onboard the spacecraft. In atmospheric pressure, operating temperature of these fuel cells is between 80°C and 90°C, with a stack efficiency of about 50%. However, under certain conditions (pressurized environment and highly concentrated electrolyte), this temperature can increase upto 250°C.
These fuel cells have several applications. Most importantly, they were used by NASA in the Apollo programs [BLU 09] and were used for the shuttles (three cells for each) to provide power of between 2 and 12 kW (with maximum power 16 kW) and an output voltage of 28–32 V. Siemens also developed alkaline fuel cells in the 1970s and 1980s, including a 20 kW cell for use in submarines. The power of alkaline fuel cells ranges between 1 and 100 kW.
Unlike platinum-based PEM fuel cells, alkaline fuel cells have the advantage of using nickel-based anode catalysts and active coal-based cathode catalysts, thereby reducing production costs.
The primary drawback of this type of fuel cell is its sensitivity to carbon monoxide. This sensitivity implies that alkaline fuel cells require an advanced hydrogen purification process (ensuring the total elimination of carbon monoxide) if this hydrogen is acquired through steam reformation of a hydrocarbonated fuel. For this reason, among others, AFC are infrequently used for transportation applications. Another reason is the fact that the electrolyte is a liquid and corrosive, and could leak under the conditions commonly found in transportation (vibration, acceleration, etc.).
1.2.3. Phosphoric acid fuel cells (PAFC)
Phosphoric acid fuel cell technology is more mature in terms of its development and commercialization. Indeed, there are stationary PAFC installations of upto 50 MW, and about 200 testing sites have been operational worldwide. Cogeneration (the simultaneous production of both electricity and heat) is the main advantage of phosphoric acid fuel cells. The power range of PAFC is between 200 kW and 50 MW. Its operating temperature is between 180 and 210°C.
Since phosphoric acid fuel cells use liquid electrolytes, they have drawback...
Table of contents
- Cover
- Title Page
- Copyright
- Introduction
- Nomenclature
- PART 1: State of the Art: Of Fuel Cells Modeling
- PART 2: Modeling of the Proton Exchange Membrane Fuel Cell
- PART 3: 1D Dynamic Model of a Nexa Fuel Cell Stack
- Bibliography
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Proton Exchange Membrane Fuel Cells Modeling by Fengge Gao,Benjamin Blunier,Abdellatif Miraoui,Fei Gao in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Power Resources. We have over one million books available in our catalogue for you to explore.