
eBook - ePub
Organic Reaction Mechanisms 2007
An annual survey covering the literature dated January to December 2007
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Organic Reaction Mechanisms 2007
An annual survey covering the literature dated January to December 2007
Book details
Book preview
Table of contents
Citations
About This Book
Organic Reaction Mechanisms 2007, the 43rd annual volume in this highly successful and unique series, surveys research on organic reaction mechanisms described in the available literature dated 2007. The following classes of organic reaction mechanisms are comprehensively reviewed:
- Reaction of Aldehydes and Ketones and their Derivatives
- Reactions of Carboxylic, Phosphoric, and Sulfonic Acids and their Derivatives
- Oxidation and Reduction
- Carbenes and Nitrenes
- Nucleophilic Aromatic Substitution
- Electrophilic Aromatic Substitution
- Carbocations
- Nucleophilic Aliphatic Substitution
- Carbanions and Electrophilic Aliphatic Substitution
- Elimination Reactions
- Polar Addition Reactions
- Cycloaddition Reactions
- Molecular Rearrangements
An experienced team of authors compile these reviews every year, so that the reader can rely on a continuing quality of selection and presentation.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Organic Reaction Mechanisms 2007 by A. C. Knipe in PDF and/or ePUB format, as well as other popular books in Scienze fisiche & Chimica organica. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
Reactions of Aldehydes and Ketones and their Derivatives
Formation and Reactions of Acetals and Related Species
Reactions of Glucosides and Nucleosides
Reactions of Ketenes
Formation and Reactions of Nitrogen Derivatives
Imines: Synthesis, Tautomerism, Catalysis
The Mannich and Nitro-Mannich Reactions
Addition of Organometallics
Other Alkylations, Arylations, and Allylations of Imines
Reduction of Imines
Iminium Species
Other Reactions of Imines
Oximes, Hydrazones, and Related Species
C–C Bond Formation and Fission: Aldol and Related Reactions
Regio-, Enantio-, and Diastereo-selective Aldol Reactions
Mukaiyama and Vinylogous Aldols
The Aldol-Tishchenko Reaction
Nitrile/Nitro/Nitroso Aldols
Other Aldol-type Reactions
Pinacol-type Coupling
The Baylis-Hillman Reaction and its Aza and Morita Variants
Allylation and Related Reactions
Alkynations
Michael Additions
Other Addition Reactions
General and Theoretical
Addition of Organozincs
Addition of Other Organometallics
Grignard-type Reactions
The Wittig Reaction
Hydrocyanation and Cyanosilylation
Hydrosilylation and Hydrophosphonylation
Miscellaneous Additions
Enolization and Related Reactions
Oxidation and Reduction of Carbonyl Compounds
Regio-, Enantio-, and Diastereo-selective Reduction Reactions
Oxidation Reactions
Other Reactions
References
Formation and Reactions of Acetals and Related Species
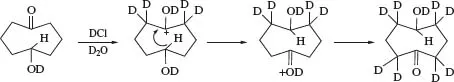
An acid-catalysed 1,5-hydride shift via a tight six-membered ring transition state, of similar overall conformation to the starting structure, has been proposed to account for the deuterium exchange of eight out of twelve methylene hydrogen atoms of 5-hydroxycyclooctanone under acidic (Scheme 1) and basic conditions in D2O, as revealed by 1H NMR measurements.1 The reaction has been analysed by quantum chemical calculations and activation barriers have been determined for the catalysed and uncatalysed reactions.
SCHEME 1
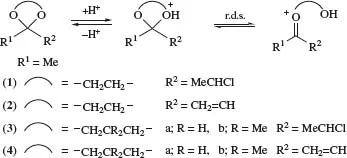
Conformational, stereoelectronic, and resonance effects on the A1 mechanism have been invoked to explain the effects of ring size and substituents on rates of acid-catalysed hydrolysis of five- and six-membered ring cyclic diol-derived ketone acetals in mixtures of THF-d8 and H2O with DCl. The results for (1)–(4) reveal resonance effects for (2) and (4) and substantial stereoelectronic effects on ring conformation whereby alignment of the equatorial or pseudo-equatorial lone pair with the σ* orbital of the breaking C-O bond can explain why a five-membered ring ethanediol-derived acetal strongly resembles a six-membered ring 2,2-dimethylpropanediol-derived acetal and why the corresponding cyclic propanediol-derived acetals hydrolyse faster than both of these.2
SCHEME 2
Chemoselective, quantitative transacetalization of aldehyde O,O-and O,S-cyclic acetals into the corresponding S,S-acetals in the presence of ketones or their acetals and oxathioacetals has been reported.3 The reactions were promoted using RSH or HSCH2CH2SH in DMF at room temperature, with cyanuric acid as the catalyst.
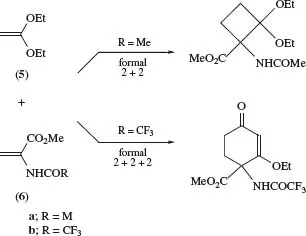
Ketene diethyl acetal (5) has been found to undergo Michael–Dieckmann-type reactions with 2-acylaminoacrylates (6a) and (6b)to give formal 2 + 2- and 2 + 2 + 2-cycloaddition products, respectively (Scheme 3).4 The results have been interpreted theoretically in terms of a polar stepwise mechanism.
SCHEME 3
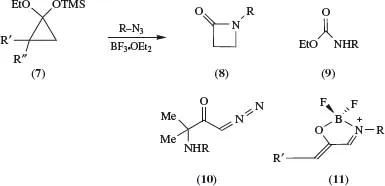
A study of the Lewis acid-mediated reactions of cyclopropanone acetals (7) with alkylazides has established that the product(s) obtained are markedly dependent on ring substituents R′, R′′, giving (8) and (9) [from azide addition to the carbonyl, followed by ring expansion or rearrangement, respectively], where R′, R′′ = H,H, (10) [from C(2)–C(3) bond cleavage of the corresponding cyclopropanone, giving oxyallyl cations that are captured by azides], where R′, R′′ = Me,Me, (11) [also the result of C(2)–C(3) bond rupture, azide capture, and loss of nitrogen] where R′, R″ = Ar,H, and (8) and (11), where R′, R′′ = «-C6H13,H. Reasons for the contrasting behaviour have been discussed.5
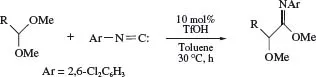
The Brønsted acid-catalysed formal insertion of an isocyanide into a C-O bond of a diverse array of acyclic and cyclic acetals (Scheme 4) can be achieved in the presence of nitro, cyano, halogen, ester, and alkoxy groups, but the course of the reaction is highly dependent on the structure of the isocyanide; an electron–deficient aryl isocyanide is required to obtain the monoinsertion product, otherwise double insertion of two aryl isocyanide molecules may occur.6 The reaction of t-octyl isocyanide also induces a double incorporation, but the subsequent acid-mediated fragmentation leads to the 2-alkoxyimidoyl cyanide. The monoinsertion products, a-alkoxyimidates, can readily be hydrolysed to a-alkoxy esters, realizing the formyl carbonylation of an acetal.
SCHEME 4
A detailed kinetic study of the pH dependence of the multistate reaction of 6-hydroxy-4′-(dimethylamino)flavylium hexafluorophosphate in aqueous solutions has revealed relatively slow hydrative formation of intermediate hemiketal species, from which cis-and trans-chalcones are obtained.7 Comparison with other flavylium compounds shows that the hydration process is affected by the amino group and that the hydroxyl group is implicated in tautomerization and isomerization reactions.
An experimental and computational (quantum chemical and FMO) study of ring-chain tautomerism of simplified analogues of oxidized and reduced isoniazid–NAD(P) adducts has identified when cyclic hemiamidal and keto-amide chain forms will predominate, respectively; the dependence on the aryl ring and on solvent polarity has been discussed.8
Pyrrolidine (20 mol%)-catalysed aldol reaction of trifluoroacetaldehyde ethyl hemiacetal with ketones or aldehydes at room temperature has been shown to afford the aldol products in good to excellent yields (up to 95%) and with much higher catalytic activity than piperidine. It is suggested that the reaction proceeds by rate-determining formation of intermediate enamine which is present in extremely low concentration during the reaction; the asymmetric aldol reaction with cyclohexanone catalysed by L-proline derivatives was also discussed.9
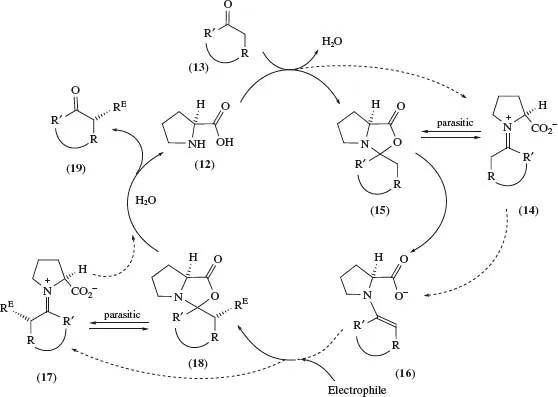
Scheme 5 depicts the generally accepted cycle (via the ‘outer route’ depicted by the dashed arrows) of proline-catalysed reactions of aldehydes and ketones with electrophiles, in which oxazolidinones (15) and (18) appear to play a role only as ‘parasitic’ species not involved in any steps for formation of product or reactive intermediates. A detailed study of oxazolidinones has now revealed direct evidence of the two hitherto putative participants [iminium ion (14) and enamine (16)] in this catalytic cycle.10 However, the commonly accepted mechanism of the stereoselective C–C or C–X bond-forming reaction with the electrophile has been challenged. An alternative model has been proposed, whereby oxazolidinones play a pivotal role in the regio-and diastereo-selective formation of the intermediate enamino acid (16) [from (15) by elimination] and in the subsequent reaction with an electrophile [as the product (18) of trans-addition with lactonization].
SCHEME 5
Reactions of Glucosides and Nucleosides
The β/α selectivity found on coupling alcohols [on preactivation with 1-benzene-sulfinylpiperidine and (CF3SO3)2O] with a series of 4,6-O-benzylidene-protected 2-O-benzyl gluco- and manno-pyranosyl thioglycosides, bearing 2-deoxy-2-fluoro and 3-deoxy-3-fluoro substituents, was found in all cases to be lower than for the corresponding simple 4,6–O-benzylidene 2,3-di-O-benzyl gluco- and manno-pyranosyl thioglycosides.11 The high β-selectivity observed for 4,6-O-benzylidene 2,3-di-O-benzylmannopyranosyl donors has therefore been ascribed to compression of the O(2)–C(2)–C(3)–O(3) torsion angle on going from the intermediate covalent glycosyl triflate to the oxacarbenium ion, rather than to the electron-withdrawing effect of the C(3)-O(3) bond.
Using three approaches (LFER analysis, X-ray crystallography, and computational modelling) it has been shown that NAG–thiazoline is a transition-state (TS) analogue for the human O-GlcNAcase-catalysed hydrolysis of β-glucosaminides whereas PUGNAc is either a poor TS analogue or a serendipitous binding inhibitor.12 The study suggests that glycosidase inhibitors should be designed to exploit the late transition state poise of the O-GlcNAse-catalys...
Table of contents
- Cover
- Title page
- Copyright page
- Contributors
- Preface
- 1. Reactions of Aldehydes and Ketones and their Derivatives by A. C. Knipe
- 2. Reactions of Carboxylic, Phosphoric, and Sulfonic Acids and their Derivatives by C. T. Bedford
- 3. Oxidation and Reduction by R. N. Mehrotra
- 4. Carbenes and Nitrenes by E. Gras
- 5. Nucleophilic Aromatic Substitution by M. R. Crampton
- 6. Electrophilic Aromatic Substitution by R. G. Coombes
- 7. Carbocations by R. A. McClelland
- 8. Nucleophilic Aliphatic Substitution by K. C. Westaway
- 9. Carbanions and Electrophilic Aliphatic Substitution by M. L. Birsa
- 10. Elimination Reactions by M. L. Birsa
- 11. Addition Reactions: Polar Addition by P. Kocovsky
- 12. Addition Reactions: Cycloaddition by N. Dennis
- 13. Molecular Rearrangements: Part 1. Pericyclic Reactions by S. K. Armstrong
- 14. Molecular Rearrangements: Part 2. Other Reactions by J. M. Coxon
- Author Index
- Subject Index