
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Concise Physical Chemistry
About this book
This book is a physical chemistry textbook that presents the essentials of physical chemistry as a logical sequence from its most modest beginning to contemporary research topics. Many books currently on the market focus on the problem sets with a cursory treatment of the conceptual background and theoretical material, whereas this book is concerned only with the conceptual development of the subject. Comprised of 19 chapters, the book will address ideal gas laws, real gases, the thermodynamics of simple systems, thermochemistry, entropy and the second law, the Gibbs free energy, equilibrium, statistical approaches to thermodynamics, the phase rule, chemical kinetics, liquids and solids, solution chemistry, conductivity, electrochemical cells, atomic theory, wave mechanics of simple systems, molecular orbital theory, experimental determination of molecular structure, and photochemistry and the theory of chemical kinetics.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER 1
IDEAL GAS LAWS
In the seventeenth and eighteenth centuries, thoughtful people, influenced by the success of early scientists like Galileo and Newton in the fields of mechanics and astronomy, began to look more carefully for quantitative connections among the phenomena around them. Among these people were the chemist Robert Boyle and the famous French balloonist Jacques Alexandre César Charles.
1.1 EMPIRICAL GAS LAWS
Many physical chemistry textbooks begin, quite properly, with a statement of Boyle’s and Charles’s laws of ideal gases:

and

The constants k1 and k2 can be approximated simply by averaging a series of experimental measurements, first of pV at constant temperature T for the Boyle equation, then of V/T at constant pressure p for Charles’s law. All this can be done using simple manometers and thermometers.
1.1.1 The Combined Gas Law
These two laws can be combined to give a new constant

Subsequently, it was found that if the quantity of gas taken is the number of grams equal to the atomic or molecular weight of the gas, the constant k3, now written R under the new stipulations, is given by

For the number of moles of a gas, n, we have

The constant R is called the universal gas constant.
1.1.2 Units
The pressure of a confined gas is the sum of the force exerted by all of the gas molecules as they impact with the container walls of area A in unit time:

The summed force f is given in units of newtons (N), and the area is in square meters (m2). The N m− 2 is also called the pascal (Pa). The pascal is about five or six orders of magnitude smaller than pressures encountered in normal laboratory practice, so the convenient unit 1 bar ≡ 105 Pa was defined.
The logical unit of volume in the MKS (meter, kilogram, second) system is the m3, but this also is not commensurate with routine laboratory practice where the liter is used. One thousand liters equals 1 m3, so the MKS name for this cubic measure is the cubic decimeter—that is, one-tenth of a meter cubed (1 dm3). Because there are 1000 cubic decimeters in a cubic meter and 1000 liters in a cubic meter, it is evident that 1 L = 1 dm3.
The unit of temperature is the kelvin (K), and the unit of weight is the kilogram (kg). Formally, there is a difference between weight and mass, which we shall ignore for the most part. Chemists are fond of expressing the amount of a pure substance in terms of the number of moles n (a pure, unitless number), which is the mass in kg divided by an experimentally determined unit molar mass M, also in kg:1
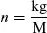
If the pressure is expressed as N m− 2 and volume is in m3, then pV has the unit N m, which is a unit of energy called the joule (J). From this, the expression

gives the unit of R as J K− 1 mol− 1. Experiment revealed that
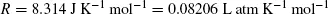
which also defines the atmosphere, an older unit of pressure that still pervades the literature.
1.2 THE MOLE
The concept of the mole (gram molecular weight in early literature) arises from the deduction by Avogadro in 1811 that equal volumes of gas at the same pressure and temperature contain the same number of particles. This somewhat intuitive conclusion was drawn from a picture of the gaseous state as being characterized by repulsive forces between gaseous particles whereby doubling, tripling, and so on, the weight of the sample taken will double, triple, and so on, its number of particles, hence its volume. It was also known at the time that electrolysis of water produced two volumes of hydrogen for every volume of oxygen, so Avogadro deduced the formula H2 O for water on the basis of his hypothesis of equal volume for equal numbers of particles in the gaseous state.
By Avogadro’s time, it was also known that the number of grams of oxygen obtained by electrolysis of water is 8 times the number of grams of hydrogen. By his 2-for-1 hypothesis, Avogadro reasoned that the less numerous oxygen a...
Table of contents
- Cover
- Half Title page
- Title page
- Copyright page
- Foreword
- Preface
- Chapter 1: Ideal Gas Laws
- Chapter 2: Real Gases: Empirical Equations
- Chapter 3: The Thermodynamics of Simple Systems
- Chapter 4: Thermochemistry
- Chapter 5: Entropy and the Second Law
- Chapter 6: The Gibbs Free Energy
- Chapter 7: Equilibrium
- Chapter 8: A Statistical Approach to Thermodynamics
- Chapter 9: The Phase Rule
- Chapter 10: Chemical Kinetics
- Chapter 11: Liquids and Solids
- Chapter 12: Solution Chemistry
- Chapter 13: Coulometry and Conductivity
- Chapter 14: Electrochemical Cells
- Chapter 15: Early Quantum Theory: A Summary
- Chapter 16: Wave Mechanics of Simple Systems
- Chapter 17: The Variational Method: Atoms
- Chapter 18: Experimental Determination of Molecular Structure
- Chapter 19: Classical Molecular Modeling
- Chapter 20: Quantum Molecular Modeling
- Chapter 21: Photochemistry and the Theory of Chemical Reactions
- References
- Answers to Selected Odd-Numbered Problems
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Concise Physical Chemistry by Donald W. Rogers in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.