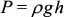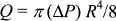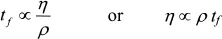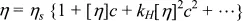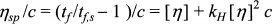![]()
Chapter 1
Introduction
A. POLYMERS AND THE IMPORTANCE OF RHEOLOGY
1. General information about the structure and properties of polymers
Polymers are generally organic and share many of the physical and chemical attributes and shortcomings, including low density, low cohesion, susceptibly to oxidative degradation, and high electrical resistance and dielectric strength. As with many organic fluids, most polymers absorb only a small amount of visible light, and are therefore colorless and transparent. If the structure of the polymer chain is regular, crystallization is possible.
The unique aspect of polymers is their high molecular weight, generally achieved by linking together organic moieties into a linear chain-like structure. Other structures are possible and useful, including random linking of the starting organic molecules into continuous net-like structures that extend indefinitely in three dimensions. Much of the commercial and research effort is focused on the linear structure, as there is some hope with this of developing a universal description. This universal description would ideally capture in a simple formula all the behavior of the polymer in terms of characteristics of the chain—length, width, stiffness, and secondary interactions with neighboring chains. Rheological behavior is one aspect where progress has been made as a result of continued work on models for the chain motions and interactions, and extensive characterization of a huge number of polymer structures. The economic motivation for this effort is that the rheology ties in closely with the physical and processing characteristics of hundreds of commercially important polymers. One can say with some validity that if polymer melts and solutions were all low in viscosity, polymer rheology would receive much less attention.
With linear polymer structures, the polymer chemist strives for high molecular weight, corresponding to long chain length, because the longest chains provide the most useful mechanical properties. Unfortunately, the longest chains also lead to the highest viscosity. Thus the chemist strives for methods to control molecular weight: high enough for good mechanical properties, and low enough for convenient processing characteristics.
Taking polyethylene as an example, at a molecular weight of 100,000 g/mol (100 kDa),* it is easy to process, with reasonable mechanical properties. At 1 MDa, the strength has improved, but processing becomes difficult, especially with techniques such as injection molding. At 10 MDa, the “ultrahigh” molecular weight range, the properties are extraordinary, but processing techniques are now confined to specialized methods, including machining of shapes. Over this 100-fold molecular-weight range, the viscosity has increased by a factor of about 400,000!
2. Rheology as a method of analysis and a quality control tool
In view of the importance of molecular weight, chemists have developed many techniques for its measurement, or estimation. While many high-accuracy instrumental techniques are now available, the standby in the laboratory is solution viscosity. Let’s examine this technique briefly, as it can provide a familiar example for introduction of some rheological terms.
The viscosity measurement is classically done by preparing several solutions of the polymer in a good solvent. The solutions should be of different concentration over a broad range. They must be free of particles, including “gel” particles that can result during the synthesis. The instrument is the familiar glass capillary viscometer. The design pictured in Figure 1-1 is often used, as the side tube ensures that the pressure at the bottom of the capillary is held constant at one atmosphere.
For the design pictured in Figure 1-1, the pressure at the top of the capillary is greater than one atmosphere because of the hydrostatic head developed by the fluid in the reservoir at the top. This pressure “head” or potential energy of the fluid in the upper bulb appears in two forms as the solution flows through the capillary: (1) kinetic energy of the exiting stream and (2) heat due to frictional (viscous) losses in the capillary. If the fluid were viscosity free, then the potential energy would be converted entirely to kinetic energy, as if the fluid were being dropped through the capillary without hitting the sides. If the viscosity is high, then the exit velocity is low and most of the energy is dissipated as heat. This is the situation that the operator wants, as it is the viscosity of the solution that is important to the analysis. Under these conditions, the flow time is proportional to the viscosity divided by the density of the fluid. Why the density? Because a high density means higher pressure at the bottom of the reservoir, and thus faster flow. Pressure beneath the surface of a quiescent fluid is given by
where P is the developed hydrostatic pressure at depth h, ρ is the density of the fluid, and g is the acceleration of gravity. The pressure-driven flow through the capillary is thus driven by a pressure that is proportional to the density of the fluid, and is resisted by the viscosity of the fluid. According to Poiseuille’s law for flow through a capillary (which we will derive later), the flow rate Q will be given by
where ΔP is the pressure drop through the capillary of length L and radius R and η is the viscosity of the fluid.† The polymer chemist measures the time tf it takes the fluid to leave the upper reservoir. This time will be lengthened by decreasing flow rate as the fluid height drops in the reservoir. However, the flow time will be proportional to the viscosity and inversely proportional to the density, i.e.,
The ratio η/ρ, called the kinematic viscosity, has dimensions of [L]2/[t] where [L] and [t] signify length and time, respectively. In SI units, this amounts to m2/s, a type of diffusivity. Mass and thermal diffusivities have the same units.
The usual way of handling the capillary experiment is to eliminate the density by dividing by the flow time of the solvent. Elimination of concentration effects is done by extrapolating to zero concentration, or by interpolating to some fixed concentration such as 0.1%. This procedure is most easily seen by examining the expected effect of polymer concentration on solution viscosity ≠ via the familiar Huggins equation:
where ηs is the solvent viscosity, c is the concentration (often g/dL) and [η] is the intrinsic viscosity. Naturally enough, the constant kH is called the Huggins’ constant. High [η] means that the polymer will have a strong effect on the solution viscosity; and, indeed, theory indicates that [η] scales as molecular weight to a power of about 0.8 for good solvents, but less for poor solvents. As Poiseuille’s equation has convinced us that the viscosity is proportional to ρtf, then
where tf,s is the flow time for pure solvent, and where the ρ’s on each side have been cancelled out. There is an assumption here that the density of all the solutions is the same, which is reasonable for dilute mixtures. The classical approach to finding [η] is to divide both sides by tf,s, subtract 1 from both sides, and finally divide by c. A plot of the modified left-hand side against c would thus give an intercept of [η] according to the relationship
Another approach, which has some statistical advantages, is to fit the observed flow times vs. concentration with the quadratic form of equation (1-5), i.e., y = a0 + a1x + a2x2, where y = tf and x = c. Once a0, a1 and a2 are found, the intrinsic viscosity is just a1/a0. Note that with this method the observation of flow time for the solvent is not necessary. With equation (1-6), a mistake in will impact directly the value of [η]; with equation (1-5), tf,s is simply another data point and counts no more than any of the others.
It should be mentioned that many routine quality-control protocols call for a single measurement of solution viscosity at a specified concentration, say, 1%. This is generally reported as simply η
sp or η
inh. The latter is determined as
Clearly, this method will work fine for distinguishing changes in molecular weight for a given polymer/solvent systems as long as the concentration is exactly right.
Solution methods for polymer analysis ha...