
eBook - ePub
Studies in Viral Ecology, Volume 2
Animal Host Systems
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
This book explains the ecology of viruses by examining their interactive dynamics with their hosting species (in this volume, in animals), including the types of transmission cycles that viruses have evolved encompassing principal and alternate hosts, vehicles and vectoring species. Examining virology from an organismal biology approach and focusing on the concept that viral infections represent areas of overlap in the ecologies of the involved species, Viral Ecology is essential for students and professionals who either may be non-virologists or virologists whose previous familiarity has been very specialized.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Studies in Viral Ecology, Volume 2 by Christon J. Hurst, Christon J. Hurst in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Microbiology. We have over one million books available in our catalogue for you to explore.
Information
Section II
Viruses of Macroscopic Animals
Chapter 5
Coral Viruses
5.1 Introduction
With up to 108 viruses mL−1 in open seawater (Bergh et al., 1989), viruses are “lubricants” of the Earth system “engine room” and are catalysts for global biogeochemical cycling by transforming planktonic cells to dissolved material. Viruses essentially act as biological transformers that accelerate the lysis of bacteria and phytoplankton (Fuhrman, 1999; Suttle, 2005; Wilhelm and Suttle, 1999). A direct consequence of this virus-induced transformation is short-circuiting the flow of carbon and nutrients to higher trophic levels and shunting the flux to the pool of dissolved and particulate organic matter. The net result is an increase in community respiration (Suttle, 2005). While such “black box” shunting exercises are extremely useful for modeling global nutrient cycling processes, they hide the enormous morphological, biological, and genetic diversity of viruses in marine systems. This is exemplified by the recent explosion of virus sequences obtained from environmental shotgun sequencing and genome sequencing projects (Breitbart and Rohwer, 2005; DeLong et al., 2006; Wilson et al., 2005b; Yooseph et al., 2007; Thurber et al., 2008). It is important to make sense of such diversity particularly in the context of global environmental change; the next big challenge for oceanic biogeochemistry is to use this genetic diversity to help understand the complexities of ecosystem functioning (Zak et al., 2006).
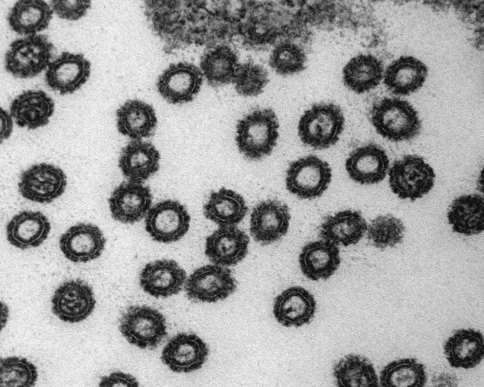
The coral reef ecosystem has an added level of complexity, almost a fourth dimension. Each coral holobiont contains a diverse assemblage of archaea, bacteria, algae, fungi, and protists as well as the coral animal (Knowlton and Rohwer, 2003). To add to the genetic and biogeochemical complexity, every microbial organism is likely to have a range of viruses that infect it. Simply looking at assemblages by transmission electron microscopy (TEM) will confirm this (Figure 5.1) (Wilson et al., 2005a; Davy et al., 2006; Davy and Patten, 2007; Patten et al., 2008a; Wilson and Chapman, 2001).
Figure 5.1 Zoanthus sp. (inset) virus-like particles (VLPs) adjacent to tentacles of a zoanthid after thermal shock. VLPs highlighted by arrows. Scale bar 1 µm. (See the color version of this figure in Color Plates section.)
While TEM allows a morphological snapshot of viruses in corals, methodological advances such as flow cytometry (Figure 5.2) and genomics are crucial to get access to numerical (Patten et al., 2006) and diversity (Dinsdale et al., 2008; Marhaver et al., 2008; Thurber et al., 2008) data, respectively, for viruses. Arguably, one of the biggest failings in coral virus research has been lack of virus isolates to work with; indeed only a few moderately successful attempts have been recorded to date (Lohr et al., 2007; Wilson et al., 2001). This largely reflects the difficulty of culturing organisms from the coral holobiont. In our experience zooxanthellae (for example) are slow growing (not conducive to virus propagation) and in general there are very few dinoflagellate viruses known (Nagasaki, 2008; Nagasaki et al., 2006). In addition, there are no known cell lines of coral animals (cnidarians) to the author's knowledge, a prerequisite for propagation of true coral viruses.
Figure 5.2 Flow cytometric analysis of seawater surrounding a nubbin of Acropora formosa following heat-shock. The virus group arrowed appeared after a 24 h heat-shock at 34 °C. (See the color version of this figure in Color Plates section.)
5.2 Why Are Viruses Not Given Prevalence in Coral Disease Diagnostics?
It is only very recently that viruses have even been considered as potential pathogens in coral systems following the observations of virus-like particles (VLPs) in sea anemones (Wilson and Chapman, 2001; Wilson et al., 2001). An incidental report of VLPs from the sea anemone Metridium senile was recorded as far back as 1974 (Chapman, 1974). M. senile was collected and thin sectioned to provide material for a review of cnidarian histology and the VLPs were mentioned in passing on Figure 5.1 of this report (also see Figure 5.3).
Figure 5.3 Electron micrograph of virus-like particles (VLPs) in a spiroblast nucleus of the plumose anemone M. senile. Scale bar approximately 500 nm (a). The VLPs are approximately 60 nm in diameter and the arrow indicates the electron dense core of approximately 40 nm of one VLP. Scale bar approximately 100 nm (b).
The simple answer is that nobody ever looks for viruses routinely in diseased corals. It is clear that they are present, since they are invariably observed if appropriate procedures are used (Cervino et al., 2004; Patten et al., 2006). Recent metagenomics work comparing diseased and nondiseased corals have also revealed a prevalence of herpesvirus-like sequences in the metagenomes (Marhaver et al., 2008; Thurber et al., 2008). So there is increasing evidence that viruses are present and likely causative agents for some of the diseases observed. Arguably, the biggest issue is that virologists, microbiologists, or molecular biologists have not traditionally addressed the problem of coral disease despite the fact that these groups of scientists have the required toolboxes to determine the role of viruses in coral reef ecosystems. This trend has started changing with more studies focusing on microbial research questions related to corals (Rosenberg et al., 2007).
According to a virologist, it seems intuitive that many coral diseases, and the largely unexplained phenomenon of coral bleaching (we know it is caused by stress factors such as increased water temperature, and the associated elimination of symbiotic zooxanthellae, but little is known of the mechanisms behind the bleaching process), in part could be caused by viruses. The coral holobiont (host organisms plus its associated microorganisms) is a soup of microbes all likely infected by viruses with a wide range of propagation strategies. These are nondisease causing viruses (that statement is clearly debatable—disease is a human perception term, seen as something wrong), which are essentially part of a natural “healthy” microbial community that provide substrate for the coral animal. Viral action is a crucial part of a healthy coral ecosystem in the same way that viruses act as “lubricants” in pelagic systems (see above). The problem arises when the system starts to break down following the appearance of spots, bands, or “rashes” on the coral system in much the same way that microbial or viral pathogens do in humans. In coral systems, problems often occur when there is an environmental insult such as temperature increase, which stresses the system resulting in biological responses (bleaching is a clear example, McClanahan et al., 2007). This may be a classic virus response, particularly by a latent virus (often termed temperate viruses), although typically latent viral activity in response to systemic stress is more prevalently studied in prokaryote virus–host systems (Edgar and Lielausis, 1964). It seems reasonable to hypothesize that latent virus systems are prevalent in coral reef systems based on observations of disease and bleaching occurrence following environmental stress conditions. One obvious assumption would be that zooxanthellae harbor latent viruses. Zooxanthellae are the “life blood” of coral reefs and anything that disrupts their ability to survive through photosynthesis and symbiosis with the coral host will have an immediate effect on the whole coral reef system (as we see with bleaching). It is a strategically relevant hypothesis that temperature induction of latent viruses can control the bleaching and/or disease process, particularly given the clear evidence that temperature increases related to climate change are having devastating effects on coral reef ecosystems (Hoegh-Guldberg et al., 2007; Carpenter et al., 2008).
5.3 Latent Coral Virus Hypothesis
With up to 108 viruses mL−1 in seawater (Bergh et al., 1989), and strong experimental evidence that these high concentrations are the result of lytic infection (Wilcox and Fuhrman, 1994), it is hardly surprising that the majority of research on marine viruses is conducted on lytic systems. Lytic infection is the process where a virus attaches to a host cell, injects its nucleic acid into the cell, and hijacks the host replication machinery to produce multiple copies of the virus. At this stage the cell bursts open (lysis), releasing viruses (and organic material) to start the cycle again.
However, there are two other generic types of virus propagation. The first is chronic infection where progeny viruses are released, typically via a budding mechanism, and the host cell, although weakened, survives the infection over several generations. The second mechanism is lysogeny (often termed latency1 in eukaryotes), where virus nucleic acid is integrated into the host genome and replicates as part of the host-cell genetic complement. The replicated virus nucleic acid is referred to as a prophage or provirus. Crucially, lytic infection can then be induced via an environmental trigger, which is typically a host stress event (e.g., elevated temperature). Although there has been research conducted specifically on lysogeny in marine prokaryotes (Weinbauer and Suttle, 1996; Wilson and Mann, 1997; Cochran et al., 1998; Jiang and Paul, 1994, 1998; McDaniel et al., 2002; McDaniel and Paul, 2005), there is a surprising paucity of data on latency in marine eukaryotic phytoplankton in the recent literature.
So is latency even important? Arguably, latency represents the most important role that viruses play in regulating the dynamics of microbial (including phytoplankton or zoxanthellae) communities in seawater and or corals. This is contrary to what most people believe, that is, their role in mortality, but latency, as the term suggests, has hidden qualities and there are strategic advantages to latent infections. Latency is a vehicle for lateral gene transfer, which fuels evolution and maintains high biodiversity of hosts. Proviruses can confer natural immunity to further infection by other lytic viruses (superinfection immunity) and they may even carry genes that are used by the host (phenotype conversion). This may either confer a selective advantage for the host (e.g., boost metabolism or confer toxicty) or the opposite is also thought to be true where a cost is associated with acquired immunity (e.g., reduced nutrient uptake efficiency). It has even been suggested that microalgae as symbionts serve as intermediary hosts for infection of other organisms (Dodds, 1979). Latency would provide the ideal refuge prior to infection of a secondary host (a secondary host could potentially be the coral or even grazing predators of the zooxanthellae).
A considerable amount of research was conducted on algal viruses in the early 1970s (largely not mentioned in recent literature), where viruses were observed in most eukaryotic algal phyla (Sherman and Brown, 1978; Dodds, 1979). Some o...
Table of contents
- Cover
- Volume 2
- Copyright
- Dedication
- Preface
- Contributors
- Attribution Credits for Cover and Spine Artwork
- Section I: An Introduction to the Structure and Behavior of Viruses
- Section II: Viruses of Macroscopic Animals
- Color Plates
- Index