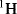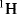![]()
Chapter 1
Interpretation of 1H NMR Spectra
As described in the preface to this book, the NMR is the most important method to identify the structure of an unknown organic compound, because the information obtained from (one dimensional and two dimensional) NMR spectra is more abundant and interpretable than that obtained by other spectroscopic methods. Since
NMR spectra have higher sensibility than other NMR spectra,
NMR spectra can be acquired more easily in some ways, and we present
NMR spectra in the first chapter of this book.
Because the
NMR spectrum can be interpreted in detail, it is possible to deduce the structure of an unknown compound, whose structure is not complex, only by using its
NMR spectrum,
NMR spectrum and the information about its molecular weight (without two dimensional NMR spectra). When we need to select the most reasonable structure from several possible structures, the
NMR spectrum of that compound can play a very important role.
Even when two dimensional NMR spectra were applied, the information, especially that from the analysis of coupled splittings in the
NMR spectrum, would still be useful to deduce an unknown structure.
The main parameters of
NMR spectra are chemical shifts, coupled constants (and splitting patterns) and peak areas. If we consider a
NMR spectrum from the viewpoint of physics, there is a fourth parameter, that is, relaxation times. However, relaxation times are short for
NMR spectroscopy. Therefore, the variation of relaxation times does not produce variations of peak areas of
NMR spectra. And relaxation times do not affect the interpretation of
NMR spectra.
The abscissa of the
NMR spectrum is the chemical shift δ, which characterizes the position in a
NMR spectrum of the peak of a functional group.
Because of coupling interactions between magnetic nuclei, peaks in the
NMR spectrum will show splittings. The splitting distance between two related split peaks is characterized by the coupling constant, measured in hertz. The magnitude of coupling constants reflects the strength of coupling interaction.
The related knowledge about the chemical shift and the coupling constant will be presented later.
The ordinate of the
NMR spectrum is the intensity of peaks. Because peaks in the
NMR spectrum have some widths, integral values of peak areas are applied as the measurements of intensities of peaks. Integral values, denoted under or beside the corresponding peaks, are proportional to the numbers of hydrogen atoms of related functional groups. The quantitative relationship of the
NMR spectrum is good with errors less than 5%.
The quantitative relationship between the integral values of peak areas and the numbers of hydrogen atoms of corresponding functional groups is important for deducing an unknown structure.
If a measured sample is a mixture, the quantitative ratio of components can be obtained from the quantitative relationship.
By using the quantitative relationship in
NMR spectroscopy, some important results can be obtained. For example, the averaged additional number of ethylene oxides, n, in a non-ionic surfactant, RO(CH
2)
nH, can be measured easily by using
NMR spectroscopy when we analyze this kind of surfactant. And the averaged value of n is more important than individual numbers which participate in average calculation for evaluating the character of this kind of surfactant. Otherwise, if we apply thin layer chromatography to analyze the surfactant, after its development by thin layer chromatography, we will get a series of spots on the thin plate. Each spot corresponds to a particular additional number and all numbers form a normal distribution shape. In this case, an average number is more important than these individual numbers which participate in average calculation.
The
spectrum of Compound
C1-1 is shown in
Figure 1.1.
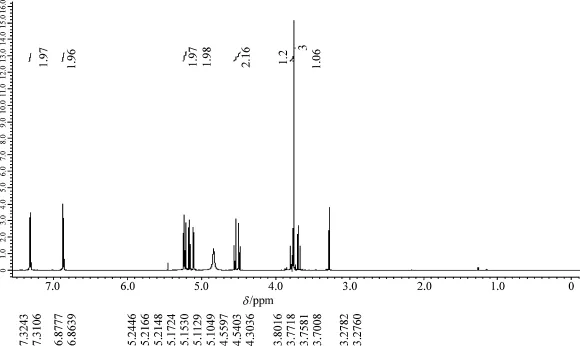
From
Figure 1.1 we know that the abscissa of the
spectrum is the chemical shift whose accurate values are denoted under (or above) corresponding peak sets. The ordinate of the
spectrum is the peak intensity. The integral values which show the areas of peak sets are denoted above (or under) corresponding peak sets.
There are split peak sets in the
spectrum. Because split distances are measured in Hz, the higher the frequency of the NMR spectrometer, the shorter the split dist...