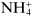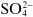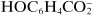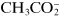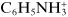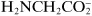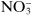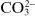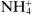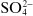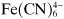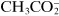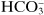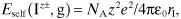![]()
PART I: FUNDAMENTALS
CHAPTER 1
ION PROPERTIES
YIZHAK MARCUS
1.1 IONS AS CHARGED PARTICLES
Ions are defined as particles that carry electrical charges. Condensed phases, solids and liquids, are electrically neutral; that is, ions exist in them in combinations of positively and negatively charged particles—cations and anions, respectively—that may be bound or relatively free to migrate. An electrolyte is a neutral combination of cations and anions that can exist as a chemical substance capable of dissociating into its constituent ions in a suitable environment, for example, in an aqueous solution.
Ions may be monatomic (such as Na
+ or Br
−), may consist of a few atoms (such as
or
) or even considerably more than a few (such as
or (C
4H
9)
4N
+), or be much larger, consisting of very many atoms. In some of such cases, they may be referred to as polyions, constituting the dissociated part of polyelectrolytes. Some polypeptides, proteins, nucleic acids, and similar biological moieties, but also suitable synthetic molecules, are examples of polyelectrolytes. Throughout this chapter, a generalized ion is designated by I
z±, but when used as a subscript the charge is dropped, and
I symbolizes a quantity pertaining to an ion.
Some substances that are not dissociated in solution, but are capable of donating a hydrogen ion to a basic environment, thus turn into an anion—these are weak acids, such as acetic acid, CH
3COOH forming
. Other substances are able to add on a hydrogen ion in an acidic medium and turn into a cation—these are weak bases, such as aniline, C
6H
5NH
2 forming
. A special category consists of zwitterions: These turn into anions or cations depending on the pH of the medium (hydrogen ion deficiency or excess), an example being glycine,
+H
3NCH
2COO
−, turning into
+H
3NCH
2COOH in acidic media and into
in basic ones. The properties discussed in this chapter pertain to ions that have been formed either by strong electrolytes directly on their dissolution or by weak electrolytes in suitable media.
1.1.1 Isolated Ions
Isolated ions may be regarded as existing in an ideal gaseous state, that is, devoid of interactions with other particles or their surroundings in general. Some quite large ions are produced in mass spectrometers, but commonly isolated ions consist of relatively few atoms. They may, however, be the centers of clusters consisting of the ion proper surrounded by a small number of solvent molecules.
The primary characteristics of isolated ions are the amount of electrical charge they carry, their mass, their shape, and their size. The amount of charge is given in terms of a multiple, zI, of the elementary units of the charge of a proton (positive) or an electron (negative), namely e = 1.60218 × 10−19 C. Within the scope of this book, the absolute values of zI for isolated ions range from 1 to 4 for monatomic ones and possibly somewhat larges for some complex ions. Highly ionized atoms that may be produced artificially or result from nuclear reactions are not considered here.
The mass of isolated ions is generally specified per mole of ions; a mole consists of a very large number of individual particles, Avogadro’s number: NA = 6.02214 × 1023 mol−1. The unit of molar mass, MI, is kg mol−1, but generally, MI is given in g mol−1.
The shape of monatomic ions is, of course, strictly spherical when isolated, but they may be deformed slightly by external forces (strong electrical fields). Ions that consist of several atoms may have any shape, but common ones are planar (
,
), tetrahedral (
,
), octahedral (
), elongated (SCN
−), or more irregular (
,
). The sizes of ions in the isolated state, however, are difficult to specify because the electrons in their periphery extend indefinitely around the inner electronic shells and the nuclei of the atoms.
An attribute of a generalized isolated ion I with charge z+ or z− (Iz±,g) is its self-energy that is due to its charge. Per mole of isolated ions, this self-energy is
where ε0 = 8.85419 × 10−12 C2 J−1 m−1 is the permittivity of free space, and rI is the radius of the ion. Since, however, as stated above the size of an isolated ion is an ill-defined quantity, so must be its radius, hence the self-energy. Still, this concept is employed in the discussion of the hydration of ions in Section 1.3.4.
Other thermodynamic quantities that pertain to isolated ions, on the other hand, are well defined. The standard molar Gibbs energy and the enthalpy of formation, ΔfG°(Iz±,g) and ΔfH°(Iz±,g), of many ions from the elements in their standard states, and the standard molar entropy and constant-pressure heat capacity, S°(Iz±,g) and CP°(Iz±,g), all at temperature T = 298.15 K have been reported in the National Bureau of Standards (NBS) tables,1 with values for additional ions being included in the book by Marcus.2 The standard molar volume of an isolated ion is a trivial quantity, the same for all ions: V°(Iz±,g) = RT/P° = 0.02479 m3 mol−1 at 298.15 K, where R = 8.31451 J K−1 mol−1 is the gas constant and P° is the standard pressure of 100 kPa.
The ionization process leading from an atom, a radical, or a molecule to a cation requires the investment of energy, expressed by the ionization potential, ΣIp. The sum sign, Σ, is used for the ionization potential Ip because this process of ionization can proceed in several stages up to the finally produced positive ion, the cation. The electron capture by an atom, a radical, or a molecule to form an anion releases energy that is expressed as the electron affinity, EA, of such a moiety. On the other hand, the capture of an electron by a negative ion, an anion, is a very unlikely event, so that only a single electron may generally be added to a neutral species in the EA process. These energies, in electron volt units (1 eV/particle = 96.483 kJ mol−1), have been reported for many ions in the aforementioned book2 and are shown in Table 1.1 for some ions.
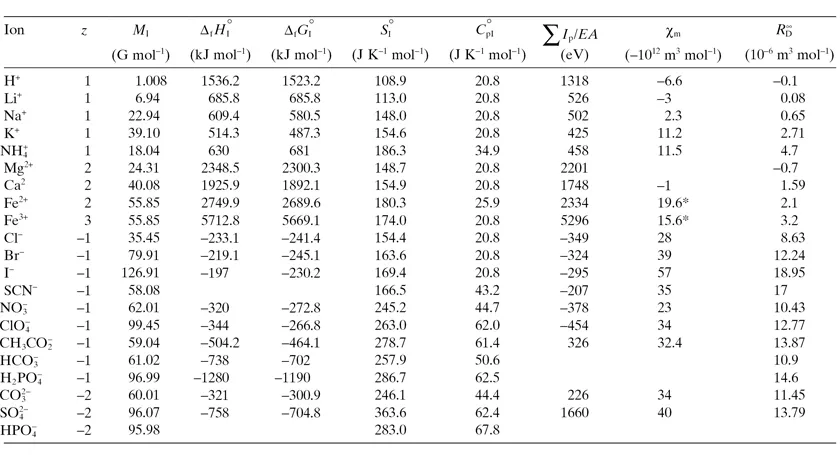
TABLE 1.1. Properties of Some Isolated Ions2
There are two further relevant properties of ions that depend little, if at all, on whether the ion is isolated in the ideal gas phase or is present in a condensed phase, a solid or a liquid. These are magnetic susceptibility, χI, and polarizability αI (or the corresponding molar refractivity, RI∞). Most ions are diamagnetic; that is, they are repulsed out from a magnetic field. Exceptions are ions that have an unpaired electron in their electronic shells: These are paramagnetic. The molar magnetic susceptibilities, χIm, range from a...