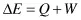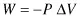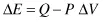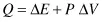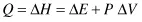![]()
1
INTRODUCTION
Talking and contention of Arguments must soon be turned into labours; all the fine dreams of Opinions and universal metaphysical natures, which the luxury of subtle brains has devised would quickly vanish and give place to solid Histories, Experiments and Works.
Hooke (1665)
The microcalorimetry of biological molecules is attracting increasing attention for several reasons. First, it was finally realized that proteins and nucleic acids, consisting of thousands of atoms participating in thermal motion, represent individual quasi-macroscopic systems. Correspondingly, they are usually called macromolecules. As with other macroscopic systems, understanding individual macromolecules requires knowledge of their thermodynamics, since that determines their most general properties.
Second, the thermodynamics of biological macromolecules is expected to be very abnormal because of the unusual spatial organization of these objects: every atom in their structure occupies a definite place, as in a crystal—but in contrast to a crystal these macromolecules have no symmetry and no periodicity in the disposition of their atoms. Such ordered aperiodic macroscopic systems have never before been dealt with in physics. Therefore, one cannot a priori predict the thermodynamic properties of biological macromolecules. In consequence, without knowing their thermodynamics one cannot engineer new macromolecules with defined properties. Without knowledge of their energetic basis, all discussion of the principles of organization of these macromolecules, of the mechanism of their formation and the stabilization of their three-dimensional structure, and therefore of their function (which assumes certain rearrangements of their structure), is mere speculation. This has become apparent only after many years of unsuccessful attempts to solve these problems by just analyzing the known structures of macromolecules. This failure has made it clear that structural information represents only one facet of a macromolecule; the other facet is its energetic basis, that is, its thermodynamics. These two fundamental information sets cannot be deduced from one another: each has to be obtained experimentally using very different methods.
Third, new and efficient experimental methods have been developed to obtain the necessary thermodynamic information on individual macromolecules in solution. Of special importance has been the development of supersensitive calorimetric instruments, isothermal reaction and heat capacity microcalorimeters, for studying the thermodynamic properties of biological macromolecules to measure the energetic bases of these molecular constructs. These properties of individual macromolecules need to be studied in highly dilute solutions—using, moreover, minimal quantities of these expensive objects: this has required especially sensitive and precise instruments.
In this book we start by reminding readers of the basics of thermodynamics useful for calorimetry and by giving relevant physicochemical information on the aqueous solutions of organic compounds. Then we describe the calorimetric techniques used for thermodynamic studies of biological macromolecules: the instruments for measuring the heat effects of various processes, namely, the heats of isothermal reactions between various reagents, the heats of temperature-induced changes in the samples being studied, that is, the heat capacities at constant pressure, and the heats associated with the pressure-induced changes at constant temperature. Calorimetry is a classical method that has been used extensively in science for a long time. However, studies of the thermodynamics of biological macromolecules, which are available in very limited amounts and can be studied only in highly dilute solutions, required development of supersensitive calorimetric instruments—microcalorimeters and even nanocalorimeters—to measure heats of isothermal reaction (isothermal titration nanocalorimeter), heat capacities over a broad temperature range (scanning nanocalorimeter), and pressure effects (pressure perturbation nanocalorimeter). Chapter 3 gives advice on how to use these techniques effectively in experiments with biological macromolecules, that is, proteins, nucleic acids, and their complexes.
Chapter 4 condenses general information on the structure of biological macromolecules—proteins and nucleic acids—to focus attention on the key thermodynamic problems relating to their structure. The results of calorimetric studies of various types of biological macromolecules and their complexes are then considered in the following chapters. We start from the two simplest, but highly important and far from fully understood, structural elements: the α and polyproline helices and their complexes, the α-helical coiled-coil and the polyproline coiled-coils. We then continue with more complicated macromolecular formations: small globular proteins; multidomain proteins and their complexes, particularly with DNA; and finally nucleic acids themselves. As will be seen, these calorimetric studies have led to serious reconsideration of many widely accepted dogmas concerning the roles of hydrogen bonding, hydrophobic interactions, and water in the formation of macromolecular structures.
Finally, I thank all my collaborators who worked with me during almost half a century on creation of a new experimental technique, microcalorimetry, and developing with such instruments a new field in experimental biophysics—the energetics of biological macromolecules. Among my numerous collaborators I have to mention particularly Vincent Cavina, Colyn Crane-Robinson, Anatoly Dragan, Vladimir Filimonov, Ernesto Freire, Hans Hinz, Nick Khechinashvili, George Makhatadze, Leonid Medved, Jamlet Monaselidze, Valery Novokhatni, Valerian Plotnikov, Wolfgang Pfeil, Sergei Potekhin, George Privalov, Oleg Ptitsyn, Rusty Russel, Tamara Tsalkova, and Paul Vaitiekunas. I have to mention specially my late friends who stimulated my involvement in studying the thermodynamics of biological macromolecules: Chris Anfinsen, John Edsall, Stanley Gill, Julian Sturtevant, and Jeffries Wyman.
I thank Thermo Analytical Instruments for their excellent manufacture of the calorimeters designed by my group and for providing photos of their parts for this book. The availability of these supersensitive instruments, nanocalorimeters, has opened a wide prospect for the experimental investigation of the thermodynamics of biological macromolecules and their complexes.
![]()
2
METHODOLOGY
2.1. THERMODYNAMIC BASICS OF CALORIMETRY
2.1.1. Energy
Energy is one of the most abstract notions. The energy conservation law is one of the greatest generalizations in science:
Energy does not disappear or appear; it only changes its form and appears in the form of mechanical energy, thermal energy, electrostatic energy, and so on.
We cannot sense energy, cannot measure it directly, but can only judge it by the manifestation of its changes, which appear in the forms of work (W) done and heat (Q) evolved:
In the case of chemical reactions, particularly those involving proteins and those connected with changes of protein structure, the mechanical work done is associated with the change of volume ΔV of the system being considered; at constant external pressure P this is
If the volume of a system decreases under the process being considered (i.e., the system is compressed under external pressure), then the work done on the system is positive, and the energy of the system increases:
2.1.2. Enthalpy
Rewriting Equation (2.3) as
it appears that the heat provided to the system changes its energy and does the work. This heat could be regarded as some invisible liquid substance that is poured into the system—for a long time heat was regarded as a liquid substance, phlogiston. It was assumed that the phlogiston poured into a system raised the heat content of a system. The heat content of a system is called enthalpy and is designated by the symbol H. Thus, in providing heat to the system we are increasing its enthalpy:
Enthalpy is the energy of the extended system: it includes not only the internal energy of a system itself (ΔE) but also the energy of its surroundings; that is, it also includes the external work (P ΔV) that is done by the system or is performed on the system by its surroundings.
Since the systems with which we usually deal are in some environment, it is clear that all changes of such systems are associated with a change of this extended energy, the enthalpy. The change of enthalpy of a system is measured by the heat that is released or absorbed by the system under the process being considered. This is just what calorimetry does: measuring the heat of reaction determines the enthalpy change of the system in which the reaction takes place.
2.1.3. Temperature
Temperature is a measure of the warmth or coldness of an object with reference to some standard value. The temperature of two macroscopic systems is the same when the systems are in thermal equilibrium. There is no heat flow between the systems when they are in equilibrium, and the heat flow between them increases with increasing difference in temperature between these systems, flowing from the warmer to the cooler system.
Temperature does not depend on the size of the system; it is an intensive characteristic of the thermal state of any macro...