
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chronic Pelvic Pain
About this book
A new addition to the Gynecology in Practice series, Chronic Pelvic Pain provides a practical guide to diagnosing and treating chronic pelvic pain in women. Emphasizing diagnosis, management and psychological aspects, the book assists gynecologists to better care for their patients suffering from this condition. As a part of the series, various feature boxes are highlighted throughout. "Tips and Tricks" give suggestions on how to improve outcomes through practical technique or patient questioning. In addition, "Caution" warning boxes supply helpful advice on how to avoid problems and "Science Revisited" boxes offer quick reminders of the basic science principles necessary for understanding the presented concepts.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Neurobiology of Chronic Pelvic Pain
Introduction
The International Association for the Study of Pain defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of that damage. Chronic pelvic pain is defined as pain in the pelvis present for at least 2 weeks out of every month for at least 6 months.
Chronic pain is much more than noxious nociception; it is a complex condition with sensory, adaptive, and affective components. While acute pain has biologic utility, chronic pain does not appear to confer any evolutionary advantage.
Many of the complexities of chronic pelvic pain are born from the complex neuroanatomy of the pelvis and corresponding responses of both the peripheral and central nervous systems; therefore a thorough understanding of the neurobiology is essential.

All structures in the pelvis should be considered as potential pain generators. In addition to the uterus and adnexa, it is also important to consider the bladder, the bowel, muscles of the pelvic floor, the skin, and both the peripheral and central nervous systems as potential pain generators. When evaluating a patient with chronic pelvic pain, make a list of all the potential pain generators in the painful area and consider the possible contribution of each one. Remember that, for many patients with chronic pelvic pain, there may be more than one pain generator in addition to changes in the central nervous system that enhance pain (central sensitization).
Nociception and Pain
The nocioceptors of somatic structures (e.g. muscle, skin, and bone) are undifferentiated nerve endings of myelinated A-delta and unmyelinated C fibers.
Mechanical, chemical, or thermal stimulation results in an influx of sodium across the sodium/potassium ion channels resulting in depolarization, which converts the noxious stimulus into an electrical impulse. The wave of depolarization is transmitted along the afferent sensory neuron, where it triggers the release of excitatory neurotransmitters at the synapse with second-order neurons in laminae I–V of the dorsal horn of the spinal cord. Some afferent nerves travel up or down for several segments in the spinal cord before making contact with second-order neurons, while others synapse at their level of entry.
The muscles of the pelvic floor and the skin of the vulva are innervated by spinal segments L4–S4, although the mons and labia also receive sensory innervation from L1 and L2 via the ilioinguinal and genitofemoral nerves.
Nociceptive input from the uterus and bladder is transmitted via the sympathetic nervous system. The sympathetic afferents also transmit some sensory input from the vagina, pelvic muscles, and skin. Sympathetic sensory axons are either thinly myelinated A-delta or unmyelinated C fibers. The pelvic viscera contain three categories of nocioceptors: stimulus specific, intensity responsive, and silent. Visceral nociceptors are unresponsive to stimuli such as cutting and crushing but very responsive to distension, traction, ischemia, and inflammation. The viscera have fewer afferent neurons compared with somatic structures.
Afferent neurons from the pelvic viscera converge with sympathetic afferents from the enteric nervous system forming a series of retroperitoneal plexuses that pass through the sympathetic chain without synapsing. These afferents enter the dorsal roots at T1–L2. Like the somatic sensory afferents, the cell bodies of the visceral afferents are located in the dorsal root ganglia. These sympathetic afferents synapse with second-order neurons, primarily in laminae I, V, and X, on both sides of the spinal cord. Parasympathetic primary afferents travel from the enteric nervous system to the central nervous system (CNS) via the vagus nerve.
Somatic and sympathetic afferents both synapse on the second-order neurons of the spinothalamic tract. There are three types of spinothalamic tract neuron in the dorsal horn: low-threshold mechanoreceptors, high-threshold nociceptors, and wide dynamic range neurons (WDRs).
WDR neurons are found in laminae I, II, V, and VI of the dorsal horn. They are multireceptive, gathering somatic input from A-delta and C fibers, with some input from A-beta fibers (touch), as well as input from visceral afferents. This convergence of both somatic and visceral afferents on the same second-order neurons results in a loss of visceral specificity, contributing to the vague and poorly localized qualities of visceral pain. Convergence is also responsible for the phenomenon of referred pain.
The second-order neurons receiving afferent input cross over to the contralateral side of the spinal cord and ascend to the brain as part of the spinothalamic, spinoreticular, and spinomesencephalic tracts. When depolarization in these ascending tracts reaches the thalamus, excitatory neurotransmitters are released that trigger depolarization of third-order neurons. These neurons are the final step in relaying nociceptive input to the somatosensory cortex, where nociception is translated into pain. As each somatic afferent has cortical representation, somatic pain is well localized; however, there is no direct visceral representation in the somatosensory cortex.

Visceral pain is typically poorly localized because viscera have no direct projections to the cerebral cortex. Well localized pain is more likely to be somatic or neuropathic in origin.
Once pain is perceived by the brain, descending pathways are activated at many levels including the cortex, thalamus, periaqueductal gray matter, nucleus raphe magnus, and locus coeruleus–subcoeruleus complex. Inhibitory pathways descend in the dorsal column and stimulate inhibitory neurons in the dorsal horn, which synapse on both the primary sensory afferents the second-order dorsal horn neurons. These descending pathways release endogenous opioids, which have an antinociceptive effect, as well as inhibitory neurotransmitters such as gamma-aminobutyric acid (GABA).
Mechanisms of Chronic Pelvic Pain
The nervous system responds dynamically to pain—this is an integral part of the pain response system. When peripheral nociceptors receive a noxious stimulus of sufficient intensity, the subsequent depolarization displaces magnesium from its binding site on the N-methyl-D-aspartate (NMDA) receptors. The open NMDA receptors are now free to bind the neurotransmitter glutamate, which increases the excitability of second-order neurons. The clinical effect is that, after an initial noxious stimulus, less input is required to trigger second-order neurons. This phenomenon is termed wind-up and is a normal response with a biologic purpose: creating hypersensitivity after injury increases the likelihood that the area will be protected from reinjury.
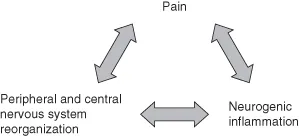
It is this ability of the nervous system to adapt in response to nocioceptive input that is the foundation for the mechanisms of chronic pain. Maladaptive responses and reorganization in both the peripheral and the CNS increase both somatosensory burden and nociceptive excitability, resulting in a self-sustaining cycle of pain and neurogenic inflammation (Figure 1.1).
Figure 1.1 Cycle of chronic pain.
Peripheral Sensitization
Peripheral sensitization is a heightened response of primary afferent nerves to nociceptive input. It plays a prominent role in the genesis and maintenance of many pelvic pain syndromes. Peripheral nociceptors may become sensitized by inflammatory neurotransmitters such as calcitonin gene-related peptide (CGRP), substance P, histamine, prostaglandins, and bradykinin. Local inflammatory changes may also activate silent nociceptor, upregulate of sodium channels, as well as trigger other genomic changes resulting in ectopic activity at the nociceptors or cell body. Endometriosis and interstitial cystitis, two conditions associated with chronic pelvic pain, have pronounced local inflammatory changes possibility facilitating peripheral sensitization. Nocioceptor sensitization has been described in women with vulvodynia.
Abnormal or excessive sprouting of peripheral nerve terminals at the site of injury or disease can also increase sensitivity to excitatory neurotransmitters, resulting in depolarization at a lower threshold or even spontaneous firing of nociceptors.
Changes in the peripheral nerves can also occur proximally at the site of synapse in the dorsal horn with second-order neurons. After peripheral nerve injury, there is greater loss of smaller C fibers than larger diameter A-beta fibers, so surviving A-beta fibers sprout new branches, making connections to second-order neurons vacated by the lost C fibers in the substantia gelatinosa (lamina II). As a result, A-beta fibers may take on a primary nociceptive role. For many patients, this is an important contributor to allodynia, the perception of light touch as pain.
Sympathetically Maintained Pain
Sympathetically maintained visceral pain is an important component of chronic pelvic pain for many patients. Like their somatic counterparts, sympathetic nociceptors may become unregulated and sensitized by injury or ongoing neurogenic inflammation, lowering the threshold for response to sympathetic stimuli such as stretching and distension. Another important mechanism of sympathetically mediated pain is activation of silent nociceptors. This phenomenon has been described in chronic bladder pain.
Abnormal sprouting is also a mechanism of sympathetically mediated pain. This may occur in neuromas at the site of injury and also in the dorsal root ganglia, where the somatic and sympathetic afferents run in close proximity. Abnormal sympathetic sprouting is reported in endometriosis implants.
Estrogen may affect vulnerability to sympathetically mediated pain. Estrogen alters micturition thresholds in rats, and in humans the menstrual cycle influences bladder pain and urgency. In animal studies, the proliferation of sympathetic neurons in the lower reproductive tract is affected by estrogen, with a significant decrease noted in ovariectomized rats.
Central Sensitization
Central sensitization refers to the changes in the CNS that facilitate, enhance, or distort pain. It is largely mediated by WDR neurons.
WDR neurons are found in lamina V. They receive input from four types of presynaptic afferents: C, A-delta, A-beta, and sympathetic. They are particularly sensitive to changes in stimulus intensity and do not normally respond to non-noxious or subthreshold stimuli. Under abnormal conditions, the WDR neurons begin to respond inappropriately to low-threshold A-beta input and may even begin to discharge spontaneously. They can also develop abnormal synapses, sprouting into other areas of the dorsal horn in response to injury and neuroinflammatory changes. Other central changes that contribute to central sensitization are recruitment of previously silent synapses in the dorsal horn and activation of glia.
Neuroinflammatory transmitters, such as CGRP, tachykinins, and glutamate, mediate changes in the dorsal horn that lead to central sensitization. Activation of NMDA receptors by glutamate, also plays a major role in the excitability of WDR neurons. Input from inhibitory interneurons (largely mediated by GABA and glycine) is also decreased, further enhancing WDR output.
Some nociceptive inputs are more likely to lead to central changes. Muscle pain is a more potent inducer of the intraspinal changes of central sensitization compared to skin. This is an important consideration as high-tone somatic dysfunction of the pelvic floor, localized myalgias, and fibromyalgia are common among patients with chronic pelvic pain. Visceral pain is also a highly effective mechanism for inducing central sensitization, producing more dorsal horn excitability when compared to cutaneous tissues.
Neuroinflammation in the spinal cord, which facilitates central sensitization, is also a key mechanism behind the multiorgan system involvement of chronic pelvic pain. Close neural connections in the sacral spinal cord are essential for the complex coordinated visceral functions of the pelvis. However, these intimate connections also allow neuroinflammation to spread from involved to uninvolved neurons via the dorsal horn. Once the end terminal of the previously uninvolved afferent is stimulated in the dorsal horn, the excitatory neurotransmitter substance P travels in a retrograde fashion down C and A-delta fibers, leading to increased expression of sodium channels and sensitization distally at the terminal nociceptors. This phenomenon is seen in animal models: rats with surgically induced endometriosis demonstrate a reduced bladder capacity, vaginal hyperalgesia, and increased visceral pain compared to animals who were subject to a sham procedure.
These central connections do more than allow pain to spread from organ system to organ system; they also allow the spread of pathology. In the murine model, an attenuated Bartha strain of pseudorabies virus (PRV) can be used to initiate a neuroinflammatory response in the spinal cord, leading not only to bladder pain, but also to inflammatory bladder pathology. As PRV is incapable of antidromic spread down either sensory or motor neurons, this effect is not mediated directly by the PRV itself but rather indirectly via neuroinflammation that spreads between shared spinal segments. This centrally-induced peripheral neurogenic inflammation is then translated into mast...
Table of contents
- Cover
- Half title page
- Title page
- Copyright page
- Series Foreword
- Preface
- Contributors
- 1 Neurobiology of Chronic Pelvic Pain
- 2 The Differential Diagnosis of Chronic Pelvic Pain
- 3 Psychogenic Causes of Chronic Pelvic Pain, And Its Impact on Psychological Status
- 4 Endometriosis: Pathogenesis and Management of Pain
- 5 Pelvic Infections and Chronic Pelvic Pain
- 6 Pelvic Congestion Syndrome
- 7 Chronic Pelvic Pain and Adhesions
- 8 Fibroids, Adenomyosis, and Chronic Pelvic Pain
- 9 Bladder Pain Syndrome and Other Urological Causes of Chronic Pelvic Pain
- 10 Chronic Pelvic Pain of Enterocolic Origin
- 11 Musculoskeletal Causes of Pelvic Pain
- 12 Dyspareunia: Causes and Treatments (Including Provoked Vestibulodynia)
- 13 Management of Chronic Pelvic Pain in the Adolescent Woman
- 14 Multidisciplinary Management of Chronic Pelvic Pain Without Obvious Pathology
- 15 The Role of Definitive Surgery in the Management of Chronic Pelvic Pain and Posthysterectomy Pain
- 16 Alternative Treatments for Chronic Pelvic Pain
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Chronic Pelvic Pain by Paolo Vercellini in PDF and/or ePUB format, as well as other popular books in Medicine & Gynecology, Obstetrics & Midwifery. We have over one million books available in our catalogue for you to explore.