
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Advanced Structural Ceramics
About this book
This book covers the area of advanced ceramic composites broadly, providing important introductory chapters to fundamentals, processing, and applications of advanced ceramic composites. Within each section, specific topics covered highlight the state of the art research within one of the above sections. The organization of the book is designed to provide easy understanding by students as well as professionals interested in advanced ceramic composites. The various sections discuss fundamentals of nature and characteristics of ceramics, processing of ceramics, processing and properties of toughened ceramics, high temperature ceramics, nanoceramics and nanoceramic composites, and bioceramics and biocomposites.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part Four: Processing and Properties of Toughened Ceramics
Chapter 10
Toughness Optimization in Zirconia-Based Ceramics
This chapter discusses major toughening mechanisms in zirconia (ZrO2) ceramics with particular attention to transformation toughening. Along with the physics and mechanics of transformation toughening, a comprehensive understanding of tetragonal ZrO2 is provided in the context of stress-induced transformation toughening. Other toughening mechanisms, for example, microcracking and ferroelastic toughening, are also discussed. The coupling of various toughening mechanisms is also addressed.
10.1 INTRODUCTION
In the ceramics literature, toughening mechanisms1 have been broadly classified into two categories: one involving a process zone around the crack tip, the other related to the bridging of crack faces by reinforcements (fibers, whiskers). The process zone mechanism is reported to enhance the material’s intrinsic toughness; examples include transformation toughening2 and microcracking. The second mechanism, that is, crack bridging, is useful in toughening ceramic matrix composites (CMCs). Among various engineering ceramics, ZrO2 is known for an excellent combination of high strength (up to 1 GPa) and good fracture toughness (up to 10 MPa m1/2), and such excellent properties are attributed to the transformation toughening mechanism. Hence, transformation toughening has attracted considerable attention during last few decades. The toughness obtainable with the transformation toughening mechanism is superior to that of the composites reinforced with particles (ZrO2-toughened Al2O3 [ZTA]) or whiskers (e.g., Al2O3/SiCW composites).3 Various ZrO2-based ceramic materials, including partially stabilized zirconia (PSZ), tetragonal zirconia polycrystals (TZPs), and fully stabilized zirconia (FSZ), as well as zirconia-toughened/dispersed ceramics (ZTC/ZDCs) are now considered as potential materials for structural, tribological, and biomedical applications.4–7 From this perspective, progress is accomplished in terms of both understanding the physics of transformation toughening3,4,8–14 and exploiting it to develop toughened materials.
The remainder of this chapter is organized into eight different sections. The characteristics of the tetragonal zirconia (t-ZrO2)-to-monoclinic zirconia (m-ZrO2) phase transformation are discussed in Section 10.2. This is followed by a short description on phase equilibria in the zirconia–yttria system in Section 10.3. Section 10.4 largely discusses the micromechanics of transformation toughening. Some theories explaining t-ZrO2 stabilization are summarized in Section 10.5. Section 10.6 discusses the production and properties of Y-TZP ceramics. In Section 10.7, the role of various microstructural variables in transformation toughening is discussed. In Section 10.8, additional toughening mechanisms are mentioned. Finally, the coupling of different toughening mechanisms is addressed in Section 10.9.
10.2 TRANSFORMATION CHARACTERISTICS OF TETRAGONAL ZIRCONIA
The crystal structures of the three major zirconia phases have been shown earlier in Chapter 2 (Fig. 2.15) and the detailed crystallographic information on different ZrO2 polymorphs can be found in Ref.15 It can be reiterated here that the cubic zirconia has the ideal fluorite structure, whereas the other polymorphs (tetragonal and monoclinic) have a distorted fluorite structure.16 It is known that undoped zirconia exhibits the following phase transitions:
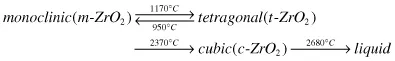
The transformations among the three polymorphs need to be considered as far as the processing and mechanical properties (strength, toughness, etc.) of zirconia ceramics are concerned. It is recognized3,4,6 that the t → m transformation in zirconia is a reversible thermal martensitic transformation, and such a transformation involves a large temperature hysteresis (∼200°C), volume change (4–5%), and considerable shear strain (14–15%). Several dopants, for example, yttria and ceria, are added to stabilize the tetragonal and/or cubic phase at room temperature (RT).4 Although c-ZrO2, t-ZrO2, and m-ZrO2 are the commonly reported phases, other zirconia phases, for example, nontransformable tetragonal (t′-ZrO2)17 and rhombohedral (r-ZrO2),16,18 can be found under certain conditions. The extreme stability of t′-ZrO2 phase is related to both higher dopant content and finer domain size (∼0.1 µm). Importantly, t′-ZrO2 is not reported to undergo phase transformation to m-ZrO2; instead the toughening in the presence of t′-ZrO2 is attributed to ferroelastic domain switching, as is discussed later.
It is known that the martensitic transformation of t-ZrO2 to m-ZrO2 is induced either during thermal cooling or by external stress application.3,4 The stress-induced martensitic transformation of t-ZrO2 enhances toughness, and this phenomenon is known as transformation toughening.
Mechanistically, the t→m ZrO2 transformation typically takes place in two major stages.16 In the first stage, the transition from tetragonal to monoclinic occurs by shear displacement of zirconium ions, and in the second stage the diffusional migration of oxygen ions to their respective sites in the monoclinic symmetry takes place. The displacement of the oxygen ions from the ideal fluorite positions has been experimentally confirmed by x-ray diffraction (XRD).19 The reverse transition of the lattice structure from m symmetry to t symmetry and the migration of the Zr+4 and O−2 ions to their respective positions are controlled by the diffusional displacements of the respective ions.20
10.3 PHASE EQUILIBRIA AND MICROSTRUCTURE
An important feature of the ZrO2–Y2O3 phase equilibria is the stabilization of the high-temperature cubic and tetragonal phases. Duwez et al. made an initial attempt to investigate the phase equilibria in the zirconia–yttria system.21 A critical thermodynamic evaluation and a modified phase diagram were later proposed by Srivastava et al.22 The widely accepted phase relationships were provided by Scott in 1975.20 Using a large high-temperature XRD (HTXRD) experiment, it was confirmed that tetragonal phase could be retained on rapid cooling and in ZrO2 ceramics with a grain size finer than a critical value (as is discussed later). The zirconia-rich part of the phase diagram is shown in Figure 10.1. The typical composition and the sintering temperature range for Y-TZP and Y-PSZ are also illustrated in Figure 10.1. The experimentally measured thermodynamic data as well as more analysis of phase stability in the ZrO2–Y2O3 system is reported elsewhere.23–25
Figure 10.1 ZrO2-rich part of the phase diagram in the ZrO2–Y2O3 system (after Scott20). The shaded regions indicate the compositions and sintering temperatures commonly used for Y-TZP and Y-PSZ.
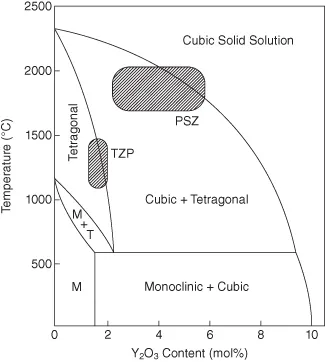
The microstructure and properties of TZP, PSZ, and ZTC can be found elsewhere.26–29 TZP contains nearly 100% tetragonal ZrO2 phase stabilized by yttria or ceria additions (Fig. 10.2a). Typical grain size of the TZPs is around 0.2–1 µm.7 As far as nomenclature is concerned, TZP ceramics are often prefixed with “Ce-” or “CeO2-” to denote ceria-stabilized ceramics, or with “Y-” or “Y2O3-” to denote yttria-stabilized ceramics. Also, a number in front of the acronym generally signifies the mole percent of dopant. For example, 3Y-TZP is the acronym for 3 mol% yttria-stabilized zirconia ceramic. The two most widely investigated TZP materials are Y-TZP and Ce-TZP, stabilized by yttria and ceria, respectively. Compared with TZP, PSZ is characterized by a coarse-grained microstr...
Table of contents
- Cover
- Title page
- Copyright page
- Dedication
- Preface
- Foreword
- About the Authors
- Section One: Fundamentals of Nature and Characteristics of Ceramics
- Section Two: Processing of Ceramics
- Section Three: Surface Coatings
- Section Four: Processing and Properties of Toughened Ceramics
- Section Five: High-Temperature Ceramics
- Section Six: Nanoceramic Composites
- Section Seven: Bioceramics and Biocomposites
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Advanced Structural Ceramics by Bikramjit Basu,Kantesh Balani in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Materials Science. We have over one million books available in our catalogue for you to explore.