
eBook - ePub
Hugo and Russell's Pharmaceutical Microbiology
This is a test
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Hugo and Russell's Pharmaceutical Microbiology
Book details
Book preview
Table of contents
Citations
About This Book
Pharmaceutical microbiology has a bearing on all aspects of pharmacy, from the manufacture and quality control of pharmaceutical products through to an understanding of the mode of action of antibiotics.
Fully revised and restructured, drawing on the contributions of subject experts, and including material relevant to the European curricula in pharmacy, the eighth edition covers:
- biology of micro-organisms
- pathogens and host response
- prescribing therapeutics
- contamination and infection control
- pharmaceutical production
- current trends and new directions
Hugo and Russell's Pharmaceutical Microbiology, a standard text for Schools of Pharmacy for seven editions, continues to be a user-friendly and authoritative guide for both students and practitioners of pharmacy and pharmaceutical microbiology.
'Highly Commended' in the Pharmacologysection of the 2012 BMA Book Awards
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Hugo and Russell's Pharmaceutical Microbiology by Stephen P. Denyer, Norman A. Hodges, Sean P. Gorman, Brendan F. Gilmore in PDF and/or ePUB format, as well as other popular books in Medizin & Pharmakologie. We have over one million books available in our catalogue for you to explore.
Information
Preface to the first edition
When we were first approached by the publishers to write a textbook on pharmaceutical microbiology to appear in the spring of 1977, it was felt that such a task could not be accomplished satisfactorily in the time available.
However, by a process of combined editorship and by invitation to experts to contribute to the various chapters this task has been accomplished thanks to the coopera- tion of our collaborators.
Pharmaceutical microbiology may be defined as that part of microbiology which has a special bearing on phar- macy in all its aspects. This will range from the manufac- ture and quality control of pharmaceutical products to an understanding of the mode of action of antibiotics. The full extent of microbiology on the pharmaceutical area may be judged from the chapter contents.
As this book is aimed at undergraduate pharmacy stu- dents (as well as microbiologists entering the pharmaceu- tical industry) we were under constraint to limit the length of the book to retain it in a defined price range. The result is to be found in the following pages. The editors must bear responsibility for any omissions, a point which has most concerned us. Length and depth of treatment were determined by the dictate of our publish- ers. It is hoped that the book will provide a concise reading for pharmacy students (who, at the moment, lack a textbook in this subject) and help to highlight those parts of a general microbiological training which impinge on the pharmaceutical industry.
In conclusion, the editors thank most sincerely the contributors to this book, both for complying with our strictures as to the length of their contribution and for providing their material on time, and our publishers for their friendly courtesy and efficiency during the produc- tion of this book. We also wish to thank Dr H.J. Smith for his advice on various chemical aspects, Dr M.I. Barnett for useful comments on reverse osmosis, and Mr A. Keall who helped with the table on sterilization methods.
W.B. Hugo
A.D. Russell
A.D. Russell
Part 1
Biology of microorganisms
1
Introduction to pharmaceutical microbiology
1 Microorganisms and medicines
2 Scope and content of the book
1 Microorganisms and m edicines
The opening paragraph of the previous edition of this book published in 2004 stated that ‘despite continuing poverty in many parts of the world and the devastating effects of HIV and AIDS, the health of the world’s population is progressively improving ’. That trend has been sustained in recent years with the number of AIDS deaths reaching a peak in 2006 and the number of new HIV infections falling 16% between 2000 and 2008. During that same period life expectancy rose in 157 out of the 193 countries reporting data to the World Health Organization and declined in only 9. Much of this improvement is due to better nutrition and sanitation, but improved health care and the greater availability of effective medicines with which to treat common human and animal diseases are also major contributing factors. Substantial inroads have been made in both the prevention and treatment of cancer, cardiovascular disease and other major causes of death in Western society, and of infections and diarrhoeal disease that remain the big killers in developing countries. Several infectious diseases have been eradicated completely, and others from substantial parts of the world. The global eradication of smallpox in 1977 is well documented, and in 2011 rinderpest, the high-mortality cattle disease which, for centuries, has contributed to poverty and famine in Africa and Asia, will also formally be declared extinct; polio and guinea-worm infection are expected to follow in the next few years.
The development of the many vaccines and other medicines that have been so crucial to the improvement in world health has been the result of the large investment in research by the major international pharmaceutical companies. This has led to the manufacture of pharmaceuticals becoming one of the most consistently successful and important industries in many countries, not only in the traditional strongholds of North America, Western Europe and Japan but, increasingly, in Eastern Europe, the Indian subcontinent and the Far East. Worldwide sales of medicines and medical devices are estimated to have exceeded $711 billion in 2007 (the latest year for which statistics are available), and in the UK pharmaceuticals was the industry sector with the largest trade surplus in 2007 having exports of £14.6 billion—a figure that translates into more than £235 000 for each employee in the industry. The growth of the pharmaceutical industry in recent decades has been paralleled by rising standards for product quality and more rigorous regulation of manufacturing procedures. In order to receive a manufacturing licence, a modern medicine must be shown to be effective, safe and of good quality. Most medicines consist of an active ingredient that is formulated with a variety of other materials (excipients) that are necessary to ensure that the medicine is effective and remains stable, palatable and safe during storage and use. While the efficacy and safety aspects of the active ingredient are within the domain of the pharmacologist and toxicolo-gist respectively, many other disciplines contribute to the quality of the manufactured product as a whole. Analytical chemists and pharmacists take lead responsibility for ensuring that the components of the medicine are present in the correct physical form and concentration, but quality is not judged solely on the physicochemical properties of the product: microorganisms also have the potential to influence efficacy and safety.
It is obvious that medicines contaminated with potentially pathogenic (disease-causing) microorganisms are a safety hazard, so medicines administered by vulnerable routes (e.g. injections) or to vulnerable areas of the body (e.g. the eyes) are manufactured as sterile products. What is less predictable is that microorganisms can, in addition to initiating infections, cause product spoilage by chemically decomposing the active ingredient or the excipients. This may lead to the product being under-strength, physically or chemically unstable, or possibly contaminated with toxic materials. Thus, it is clear that pharmaceutical microbiology must encompass the subjects of sterilization and preservation against microbial spoilage, and a pharmacist with responsibility for the safe, hygienic manufacture and use of medicines must know where microorganisms arise in the environment, i.e. the sources of microbial contamination, and the factors that predispose to, or prevent, product spoilage. In these respects, the pharmaceutical microbiologist has a lot in common with food and cosmetics microbiologists, and there is substantial scope for transfer of knowledge between these disciplines.
Disinfection and the properties of chemicals (biocides) used as antiseptics, disinfectants and preservatives are subjects of which pharmacists and other persons responsible for the manufacture of medicines should be familiar, both from the perspective of biocide use in product formulation and manufacture, and because antiseptics and disinfectants are pharmaceutical products in their own right. However, they are not the only antimicrobial substances that are relevant to medicine: antibiotics are of major importance and represent a product category that regularly features among the top five most frequently prescribed. The term ‘antibiotic ’ is used in several different ways: originally an antibiotic was defined as a naturally occurring substance that was produced by one microorganism that inhibited the growth of, or killed, other microorganisms, i.e. an antibiotic was a natural product, a microbial metabolite. More recently the term has come to encompass certain synthetic agents that are normally used systemically (throughout the body) to treat infection. The manufacture, quality control and, in the light of current concerns about resistance of microorganisms, the use of antibiotics, are other areas of knowledge that contribute to the discipline of pharmaceutical microbiology.
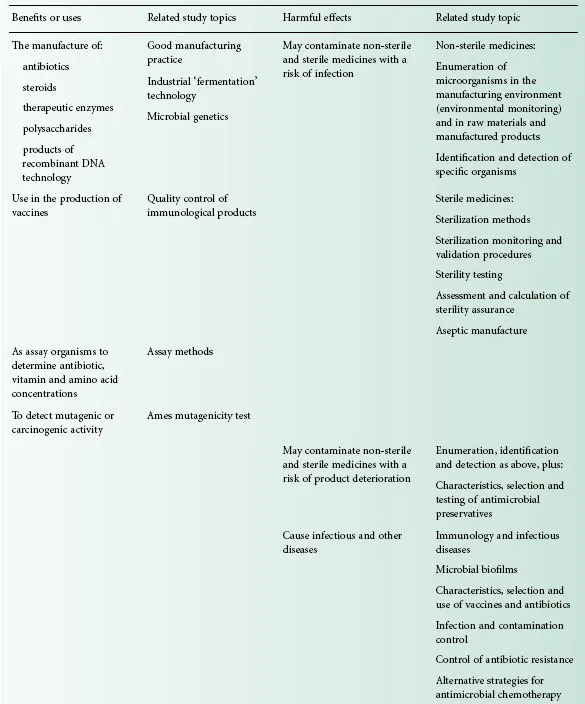
Commercial antibiotic production began with the manufacture of penicillin in the 1940s, and for many years antibiotics were the only significant example of a medicinal product that was made using microorganisms. Following the adoption in the 1950s of microorganisms to facilitate the manufacture of steroids and the development of recombinant DNA technology in the last three decades of the 20th century, the use of microorganisms in the manufacture of medicines has gathered great momentum. It led to more than 100 biotechnology-derived products on the market by the year 2000 with another 300 or more in clinical trials. While it is true to say that traditionally the principal pharmaceutical interest in microorganisms is that of controlling them, exploiting microbial metabolism in the manufacture of medicines is a burgeoning area of knowledge that will become increasingly important, not only in the pharmacy curriculum but also in those of other disciplines employed in the pharmaceutical industry. Table 1.1 summarizes these benefits and uses of microorganisms in pharmaceutical manufacturing, together with the more widely recognized hazards and problems that they present.
Table 1.1 Microorganisms in pharmacy: benefits and problems

Looking ahead to the second decade of the 21st century, it is clear that an understanding of the physiology and genetics of microorganisms will also become more important, not just in the production of new therapeutic agents but in the understanding of infections and other diseases. Genetic techniques such as ribotyping are becoming increasingly used to identify cross-infection, reduce transmission and optimize management of hospital-acquired infections, e.g. those due to Clostridium difficile, and, because of the traditional breadth of their science education and their accessibility to the public, pharmacists are not infrequently called upon to explain the terminology and concepts of genetics and other biological sciences to both work colleagues and patients. Several of the traditional diseases that were major causes of death before the antibiotic era, e.g. tuberculosis and diphtheria, are now re-emerging in resistant form—even in developed countries—adding to the problems posed by infections in which antibiotic resistance has long been a problem, and those like Creutzfeldt–Jakob disease, West Nile virus and severe acute respiratory syndrome (SARS) that have only been recognized or have changed in character in recent years.
Not only has the development of resistance to established antibiotics become a challenge, so too has the ability of microorganisms to take advantage of changing practices and procedures in medicine and surgery. Microorganisms are found almost everywhere in our surroundings and they possess the potential to reproduce extremely rapidly; it is quite possible for cell division to occur every 20 minutes under favourable conditions. These characteristics mean that they can adapt readily to a changing environment and colonize new niches. One feature of modern surgery is the ever-increasing use of plastic, ceramic and metal devices that are introduced into the body for a wide variety of purposes, including the commonly encountered urinary or venous catheters and the less common intraocular lenses, heart valves, pacemakers and hip prostheses. Many bacteria have the potential to produce substances or structures that help them to attach to, and grow as biofilms over, the surfaces of these devices, even while combating the immune system of the body. Thus, colonization often necessitates removal and replacement of the device in question—often leading to great discomfort for the patient and substantial monetary cost to the healthcare service. It has been estimated that, on average, a hospital-acquired infection results in an extra 14 days in hospital, a 10% increase in the chance of dying and an additional health-care cost per patient of between £1700 and £4120. The development of strategies for eliminating, or at least restricting, the severity or consequences of these device-related infections is a challenge for pharmacists and microbiologists within the industry, and for many other healthcare professionals.
In addition to an improved understanding of the mechanisms of antibiotic resistance, of the links between antibiotic resistance and misuse, and of the factors influencing the initiation of infections in the body, our insights into the role of microorganisms in other disease states have broadened significantly in recent years. Until about 1980 it was probably true to say that there was little or no recognition of the possibility that microorganisms might have a role to play in human diseases other than clear-cut infections. In recent years, however, our perception of the scope of microorganisms as agents of disease has been changed by the discovery that Helicobacter pylori is intimately involved in the development of gastric or duodenal ulcers and stomach cancer; by the findings that viruses can cause cancers of the liver, blood and cervix; and by the suspected involvement of microorganisms in diverse conditions like chronic fatigue syndrome and Alzheimer ’ s disease. These, and other conditions like Bell ’ s palsy, atherosclerosis and multiple sclerosis, were amongst 16 chronic diseases suspected of having infectious origins that were named in a 2005 report published by the American Academy of Microbiology.
Clearly, a knowledge of the mechanisms whereby microorganisms are able to resist antibiotics, colonize medical devices and cause or predispose humans to other disease states is essential in the development not only of new antibiotics, but of other medicines and healthcare practices that minimize the risks of these adverse situations developing.
2 Scope and content of the book
Criteria and standards for the microbiological quality of medicines depend upon the route of administration of the medicine in question. The vast majority of medicines that are given by mouth or placed on the skin are non-sterile, i.e. they may contain some microorganisms (within limits on type and concentration), whereas all injections and ophthalmic products must be sterile, i.e. containing no living organisms. Products for other anatomical sites (e.g. nose, ear, vagina and bladder) are often sterile but not invariably so (Chapter 22). The microbiological quality of non-sterile medicines is controlled by specifications defining the concentration of organisms that may be present and requiring the absence of specific, potentially hazardous organisms. Thus the ability to identify the organisms present, to detect those that are prohibited from particular product categories, and to enumerate microbial contaminants in the manufacturing environment, raw materials and finished product are clearly skills that a pharmaceutical microbiologist should possess (Chapters 2–6). So, too, is a familiarity with the characteristics of antimicrobial preservatives that may be a component of the medicine required to minimize the risk of microbial growth and spoilage during storage and use by the patient (Chapters 17 and 19).
For a sterile product the criterion of quality is simple: there should be no detectable microorganisms whatsoever. The product should, therefore, be able to pass a test for sterility, and a knowledge of the procedures and interpretation of results of such tests is an important aspect of pharmaceutical microbiology (Chapter 21). Injections are also subject to a test for pyrogens; these are substances that cause a rise in body temperature when introduced into the body. Strictly speaking, any substance which causes fever following injection is a pyrogen, but in reality the vast majority are of bacterial origin, and it is for this reason that the detection, assay and removal of bacterial pyrogens (endotoxins) are considered w...
Table of contents
- Cover
- Fmatter
- Title
- Copyright page
- List
- Preface 8
- Preface 1
- Part 1: Biology of microorganisms
- Part 2: Pathogens and host responses
- Part 3: Prescribing therapeutics
- Part 4: Contamination and infection control
- Part 5: Pharmaceutical production
- Part 6: Current trends and new directions
- Index