
Novel Concepts in Catalysis and Chemical Reactors
Improving the Efficiency for the Future
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Novel Concepts in Catalysis and Chemical Reactors
Improving the Efficiency for the Future
About This Book
The chemical process industry faces a tremendous challenge of supplying a growing and ever more demanding global population with the products we need. The average efficiency at which resources are converted into the final products is however still dramatically low. The most obvious solution is to carry out chemical conversions at much higher yields and selectivity and this is where active and selective catalysts and efficient chemical reactors play a crucial role. Written by an international team of highly experienced editors and authors from academia and industry, this ready reference focuses on how to enhance the efficiency of catalysts and reactors. It treats key topics such as molecular modeling, zeolites, MOFs, catalysis at room temperature, biocatalysis, catalysis for sustainability, structured reactors including membrane and microchannel reactors, switching from batch to continuous reactors, application of alternative energies and process intensification. By including recent achievements and trends, the book provides an up-to-date insight into the most important developments in the field of industrial catalysis and chemical reactor engineering. In addition, several ways of improving efficiency, selectivity, activity and improved methods for scale-up, modeling and design are presented in a compact manner.
Frequently asked questions
Information
- Sabatier principle and volcano curve;
- Brønsted-Evans-Polanyi (BEP) linear activation energy-reaction energy relationships;
- compensation effect in catalytic kinetics;
- micropore size dependence in zeolite catalysis;
- structure sensitivity and insensitivity in transition-metal catalysis;
- transition-state stabilization rules.




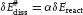
Table of contents
- Cover
- Title
- Copyright
- Preface
- List of Contributors
- 1: Molecular Catalytic Kinetics Concepts
- 2: Hierarchical Porous Zeolites by Demetallation
- 3: Preparation of Nanosized Gold Catalysts and Oxidation at Room Temperature
- 4: The Fascinating Structure and the Potential of Metal-Organic Frameworks
- 5: Enzymatic Catalysis Today and Tomorrow
- 6: Oxidation Tools in the Synthesis of Catalysts and Related Functional Materials
- 7: Challenges in Catalysis for Sustainability
- 8: Catalytic Engineering in the Processing of Biomass into Chemicals
- 9: Structured Reactors, a Wealth of Opportunities
- 10: Zeolite Membranes in Catalysis: What Is New and How Bright Is the Future?
- 11: Microstructures on Macroscale: Microchannel Reactors for Medium- and Large-Size Processes
- 12: Intensification of Heat Transfer in Chemical Reactors: Heat Exchanger Reactors
- 13: Reactors Using Alternative Energy Forms for Green Synthetic Routes and New Functional Products
- 14: Switching from Batch to Continuous Processing for Fine and Intermediate-Scale Chemicals Manufacture
- 15: Progress in Methods for Identification of Micro- and Macroscale Physical Phenomena in Chemical Reactors: Improvements in Scale-up of Chemical Reactors
- Index