![]()
1
Introduction and Technical Notes
In the year 1609, Johannes Kepler published a standard work of astronomy, the
Astronomia Nova, sev Physica Coelestis, tradita commentariis de Mortibvs Stellæ Martis: “The New Astronomy, or Celestial Physics, based on records on the Motions of the Star Mars.” In Chapter LIX (59), he summarizes what became known as Kepler’s first and second law (Figure
1.1). The heading of this chapter starts as follows:
Demonstratio, qvod orbita Martis… fiat perfecta ellipsis: “This is to demonstrate that the Martian orbit… is a perfect ellipse,” or-in today’s common phrasing of Kepler’s first law: “The planet’s orbit is an ellipse, with the Sun at one focus.” The second law states that the “line connecting the Sun and the planet sweeps out equal areas in equal time intervals.” (The third law was formulated 10 years later:
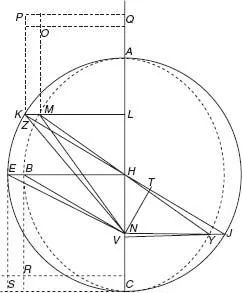
,
p = revolutionary period,
r = semimajor axis; the lower indices 1 and 2 refer to two planets.) Kepler’s pioneering mathematical treatise, based on minute observations collected by Tycho Brahe, had been a breakthrough for astronomy, and applications of his laws are still influential in modern astronomy.
A second trailblazing event 400 years ago was the discovery of what is now known as the “Galilean moons,” the four large moons of the planet Jupiter. Galileo Galilei announced the discovery of three of the Jovian moons on the 7th of January 1610 (discovery of the fourth moon followed a couple of weeks later)-according to the Gregorian calendar, which corresponds to the 28th of December, 1609, in the Julian calendar. In honor of his mentor Cosimo II de Medici, Galilei named the moons Cosmica Sidera (Cosimo’s stars), and then Medicea Sidera (stars of the Medici). Following a suggestion by Simon Mayr (or Simon Marius in the Latinized version) in 1614, the four moons were termed “Io, Europa, Ganymed atque (and) Callisto lascivo nimivm perplacvere Iovi” (… who greatly pleased lustful Jupiter [Zeus]). Simon Mayr discovered the moons independently of Galilei, but announced his discovery a day later, on the 8th of January 1610. The discovery of the moons, and realization that the moons orbit Jupiter, was a final bash against a geocentric worldview of the Universe dominating medieval times.
The two discoveries became duly commemorated in the 2009 International Year of Astronomy, which was also the year for a couple of key discoveries in astronomy, astrophysics, astrochemistry, and astrobiology: (i) detection of the first exoplanets with physical and chemical characteristics approximating those of our home planet; (ii) reinvestigation of nanosized magnetite crystals, possible biomarkers, in a Martian meteorite recovered in Antarctica in 1984 (see also Preface); (iii) discovery of the glycine precursor aminoacetonitrile (see cover of this book) in the "Large Molecular Heimat," a dense interstellar molecular cloud in the constellation of Sagittarius; (iv) the final proof that our next neighbor in the Cosmos, our Moon, contains sizable reservoirs of water, possibly of cometary origin, deposited in permanently shaded craters; and (v) location of the most distant and oldest object in the Universe, a gamma ray burst associated with a stellar-sized black hole or magnetic neutron star, which formed just 630 million years after the Big Bang, the event which is considered the hour of birth of our Universe, 13.7 billion years ago.
These are just a few selected highlights, supposed to adumbrate the scope of the present treatise, and to be addressed together with other topical and less recent events and discoveries in some detail in this book. The book will focus on aspects in astronomy related to chemistry-in stars and the interstellar medium, in the atmospheres, on the surfaces, and in subsurface areas of planets, planetoidal bodies, moons, asteroids, comets, interplanetary, and interstellar dust grains. A topical point to be covered is the query of the origin of life, either on Earth or somewhere else in our Milky Way galaxy, and the genesis of basic molecules functioning as building blocks for complex molecules associated with life and/or representing life. Along with these chemistry-related issues, general cosmological aspects related to astronomy and astrophysics, and often indispensable for an axiomatic comprehension of chemical processes, will be approached. Some knowledge of the basics of chemical (including bio- and physicochemical) coherency will be afforded to become involved: the book is designed so as to be both an introduction for the interested beginner with some basic knowledge, and a compendium for the more advanced scientist with a background in chemistry and adjacent disciplines.
Several of the crucial points covered in the present book have been treated in book publications by other authors, usually with another target course, that is, less intimately directed toward chemical and biological aspects of astronomical problems. The following glossary (sorted chronologically) is a selection of books and compendia that have animated me during the bygone two decades, and are thus recommended as "Further Reading".
– Duley, W.W., Williams, D.A. (1984) Interstellar Chemistryi Academic Press, London.
– Saxena, S.K. (ed.) (1986) Chemistry and Physics ofthe Terrestrial Planets [vol. 6 of Advances in Physical Geochemistry], Springer Verlag, Berlin.
– Lewis, J.S. (1995) Physics and Chemistry of the Solar Systemi Academic Press, San Diego. [2nd Edition (2004): Elsevier/Academic Press]
– Szczerba, R., Gorny, S.K. (eds.) (2001) Post-AGB Objects as a Phase ofStellar Evolution [vol. 265 of Astrophysics and Space Science Library], Kluwer Academic, Dordrecht.
– Clayton, D.D. (2003) Handbook ofIsotopes in the Cosmos, Cambridge University Press, Cambridge.
– Green, S.F., Jones, M.H. (eds.) (2003/04) An Introduction to the Sun and Stars, Cambridge University Press, Cambridge.
– Thielens, A.G.G.M. (2005) The Physics and Chemistry of the Interstellar Medium, Cambridge University Press, Cambridge.
– Shaw, A.M. (2006) Astrochemistry - From Astronomy to Astrobiology, John Wiley & Sons, Chichester.
– Plaxco, K.W., Gross, M. (2006) Astrobiology, The John Hopkins University Press, Baltimore.
– Kwok, S. (2007) Physics and Chemistry ofthe Interstellar Medium, University Science Books, Sausalito, CA.
– Shapiro, S.L, Teukolsky, S.A (2007) Black Holes, White Dwarfs, and Neutron Stars, Wiley VCH, Weinheim.
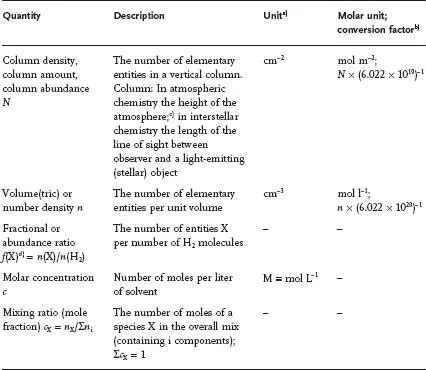
Scientists enrooted in astronomy do have their subject-specific nomenclature and system of units, which is not always easily accessible to a chemist. As an example, if it comes to the term "concentration" (of a specific species X in a mix), chemists use to think in terms of "molarity" (moles of X per liter of the mix) or "molality" (moles of X per kg), where "mole" relates to the amount of substance: 1 mole of any substance is equal to 6.022 × 1023 elementary entities. Examples for elementary entities are elementary particles (such as electrons, protons, and neutrons), atoms, ions, molecules, light quanta. In contrast, astronomers commonly refer to concentration in terms of " column density/abundance/amount, " " fractional density, " and " number/volume density, " conceptions so uncommon for chemists that they hardly do associate any perception with these quantifications. From a chemist’s point of view, column amount quoted in terms of mol m–2 (i.e., employing the units of the Système Internationale, the SI system) is "correct" [1] and has been used wherever sensibly applicable - together with the units preferred by astronomers. Table 1.1 provides an overview of conversions of units for "concentration," frequently employed in astronomical and astrophysical articles, into molar units. Conversions will also be provided in the main text wherever this appears to be reasonable.
Most of the units employed in this book are SI units. Where our conceptions from everyday experience are dominated by more classical units, both the SI and the popular units are provided. Examples are temperature (in Kelvin or degrees Celsius), pressure (in Pascal or bar), strength of the magnetic field (the
B field; in Tesla or Gauss). Distances in astronomical dimensions, when expressed in meters or 10
3 multiples thereof, are not easily handled by our spatial perception. Astronomical units (AUs), parsecs (pc), and light-years (ly), as defined in Figure
5.2 and Table
5.3, are more easily comprehended and therefore used throughout. Similarly, if it comes to "astronomical ages," years (a, derived from the Latin
annum) and multiples thereof, such as megayears (Ma = 10
6 a) and gigayears (Ga = 10
9 a) are employed rather than the SI unit "second." Finally, masses (
m, SI unit: g) are quoted, were appropriate, in
m⊕ (multiples of Earth; ⊕ is the astronomical symbol for Earth),
mJ (multiples of Jupiters) and
m (multiples of Suns;
is the symbol for the Sun). The lower case letter "m" otherwise stands for magnitude (of a star); the
capital letter M (≡ mol l
–1) denotes molarity and, in chemical equations, "metal" (all elements beyond helium), while
M ...