![]()
CHAPTER 1
INTRODUCTION
An immense amount of experimental data has been accumulated from investigations of the infrared absorption spectra and of the Raman effect in polyatomic molecules. Only an extremely small fraction of this material has been subjected to analysis, although the theoretical tools for such an analysis are quite well developed and the results which could be obtained are of considerable interest. One reason for this situation is the amount of labor required to unravel the spectrum of a complex molecule, but an additional deterrent has been the unfamiliar mathematics, such as group theory, in terms of which the most powerful forms of the theory of molecular dynamics have been couched. When only the necessary parts of these mathematical techniques are considered, the difficulty of understanding the theory of the vibrational and rotational spectra of polyatomic molecules is greatly reduced.
In this first chapter, a short general survey of the background of the subject will be given, to serve as an introduction to the more mathematical treatment which follows.
1-1. Infrared Spectra
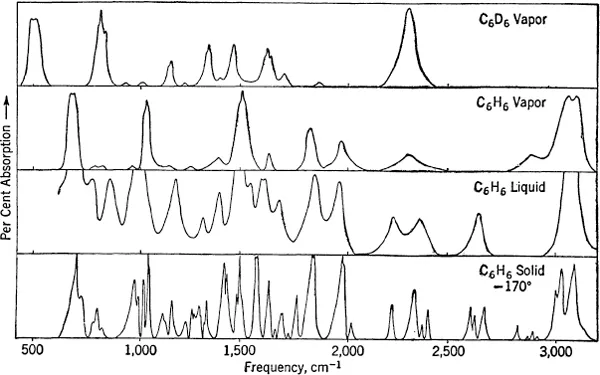
The absorption or emission spectrum arising from the rotational and vibrational motions of a molecule which is not electronically excited is mostly in the infrared region. A small molecule having an electric moment emits and absorbs light of frequency below about 250 wave numbers1 because of its rotational motion. Molecules which are absorbing or emitting 1 quantum of vibrational energy show bands in the region from about 200 to 3,500 cm−1, while bands due to several vibrational quantum jumps are detected all the way from a few hundred to many thousand wave numbers, sometimes being observable in the visible portion of the spectrum (13,000 to 26,000 cm−1). See Fig. 1-1.
Infrared spectra may be observed either in emission or in absorption, although the latter method is by far the more common. In absorption experiments light from a suitable source is passed through a tube containing the gas to be studied and thence into the spectrograph.1 If the spectrograph is of low resolving power, a series of wide bands is observed which correspond to the vibrational transitions, but if a spectrograph of higher resolution is used, these bands may break up into lines which can be correlated with the energy levels of rotation. In practice, only a few light molecules (H2O, NH3, CH4, CO2, etc.) have been observed in the infrared region with high enough resolving power to resolve the rotational structure. Figure 1-1 shows some observed spectra.
FIG. 1-1. Vibrational absorption spectra in the infrared as illustrated by benzene.
Liquids and solids are also studied, and they yield interesting results, but except in so far as they give vibrational spectra in close agreement with those found for the corresponding gases they will not be discussed in this book, in which interactions between separate molecules will be neglected.
1-2. Raman Spectra1
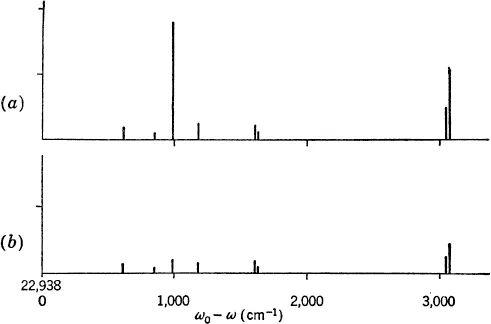
If the substance being studied (as a gas, liquid, or solid) is strongly illuminated by monochromatic light in the visible or ultraviolet region and the scattered light observed in a spectrograph,2 a spectrum is obtained (see Fig. 1-2) which consists of a strong line (the exciting line) of the same frequency as the incident illumination together with weaker lines on either side shifted from the strong line by frequencies ranging from a few to about 3,500 wave numbers. The pattern of lines is symmetrical about the exciting line except with regard to intensities, the lines on the high-frequency side being considerably weaker than the others. In fact, they are frequently too weak to be observed. The lines of frequency less than the exciting lines are called Stokes lines, the others anti-Stokes lines.
These lines differing in frequency from the exciting line, the Raman lines, have their origin in an interchange of energy between the light quanta and the molecules of the substance scattering the light. The lines which appear very near the exciting line are correlated with changes in the rotational energy states of the molecules without changes in the vibrational energy states and form the pure rotation Raman spectrum. The lines farther from the exciting line are really bands of unresolved lines and are associated with simultaneous changes in the vibrational and rotational energy states.
The frequency shifts, that is, the differences between the frequencies of the Raman lines and the exciting line, are independent of the frequency of the exciting line. A mercury arc is usually used for illumination, and there are a number of the strong mercury lines which are used to excite Raman spectra. The frequencies for a given molecule found in infrared absorption frequently agree with the frequency shifts found in the Raman effect, but this is not always true and depends on the symmetry of the molecule in a way which is now well understood.
FIG. 1-2. Plot of the Raman spectrum of benzene. The heights of the lines indicate relative intensity, (a) being the parallel spectrum and (b) the perpendicular spectrum (b/a = ρn; see Sec. 3-6). The scale is in cm−1 and the Raman shift is measured from the mercury exciting line at 22,938.04 cm−1 (4,358 A). (These data, used in the example discussed in Chap. 10, are from Angus, Ingold, and Leckie, J. Chem. Soc., 1936, p. 925.)
By polarizing the incident light or in other ways, it is possible to find the degree of depolarization of each frequency shift in the Raman spectrum, a quantity which will be important in the interpretation of the experimental results. This quantity is the ratio, for the scattered light, of the intensities of the components polarized perpendicular and parallel, respectively, to the direction of polarization of the incident illumination.
1-3. The Molecular Model
In attempting to account for the observed infrared and Raman spectra of real molecules, a certain simplified model for such molecules is adopted, and then the spectra which this model would exhibit are calculated. The specification of the model involves certain parameters such as size, stiffness of valence bonds, etc., which can be varied within limits set by other types of experimental evidence until the best agreement with experiment is obtained. An attempt is usually made to select a number of such parameters which is much smaller than the number of experimental quantities so that the success of the theory can be tested by the agreement which it provides with experiment.
The model which will be used in this book consists of particles held together by certain forces. The particles, which are to be endowed with mass and certain electrical properties, represent the atoms and are to be treated as if all the mass were concentrated at a point. It is assumed that the atoms may be electrically polarized by an external electrical field, such as that of a beam of light, and that they may or may not be permanently polarized by their mutual interactions in such a manner that the whole molecule has a resultant electric moment.1 Both the polarizability and the electric moment of the model may vary as the particles (hereafter called atoms) change their relative positions. Finally, the atoms may possess an internal degree of freedom or nuclear spin which introduces certain symmetry restrictions.
The forces between the particles may be crudely thought of as weightless springs which only approximately obey Hooke’s law and which hold the atoms in the neighborhood of certain configurations relative to one another. This picture of the forces as springs is useful for visualization, but is not sufficiently general for all cases. For example, it does not cover cases of restricted rotation about single bonds such as may occur in ethane. The nature of these interatomic forces is one of the chief problems still being studied and will be discussed in Chap. 8. The search for a potential function which involves a small number of parameters and which at the same time permits good agreement with experiment is by no means ended.
The statement that the model obeys the laws of quantum mechanics is an essential part of its specification. However, since atoms are fairly heavy particles (compared to electrons), it will sometimes be true that classical mechanics when properly used gives results which are good approximations to those of quantum mechanics.
Since the atoms of this model have been regarded as point masses with certain electrical properties, there is an apparent disagreement with the fact that many experiments require that atoms be made of electrons and nuclei. It is possible to reconcile these two points of view. If the wave equation for a molecule made up of electrons and nuclei is set up, a procedure1 exists whereby this equation may be separated into two equations, one of which governs the electronic motions and yields the forces between the atoms, whereas the other is the equation for the rotation and vibration of the atoms and is identical with the equation for the model adopted here. In principle, therefore, the forces between the atoms can be calculated a priori from the electronic wave equation, but in practice this is not mathematically feasible (except for H2), and it is necessary to postulate the forces in such a manner as to obtain agreement with experiment. Therefore, a...