
Physics of Shock Waves and High-Temperature Hydrodynamic Phenomena
- 944 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Physics of Shock Waves and High-Temperature Hydrodynamic Phenomena
About This Book
High temperatures elicit a variety of reactions in gases, including increased molecular vibrations, dissociation, chemical reactions, ionization, and radiation of light. In addition to affecting the motion of the gas, these processes can lead to changes of composition and electrical properties, as well as optical phenomena.
These and other processes of extreme conditions — such as occur in explosions, in supersonic flight, in very strong electrical discharges, and in other cases — are the focus of this outstanding text by two leading physicists of the former Soviet Union. The authors deal thoroughly with all the essential physical influences on the dynamics and thermodynamics of continuous media, weaving together material from such disciplines as gas dynamics, shock-wave theory, thermodynamics and statistical physics, molecular physics, spectroscopy, radiation theory, astrophysics, solid-state physics, and other fields.
This volume, uniquely comprehensive in the field of high-temperature gas physics and gas dynamics, was edited and annotated by Wallace D. Hayes and Ronald F. Probstein, leading authorities on the flow of gases at very high speeds. It is exceptionally well suited to the needs of graduate students in physics, as well as professors, engineers, and researchers.
Frequently asked questions
Information
VI. Rates of relaxation processes in gases
1. Molecular gases
§1. Establishment of thermodynamic equilibrium
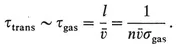
Table of contents
- Title Page
- Copyright Page
- Dedication
- Preface to the Dover Edition
- Editors’ foreword
- Preface to the English edition
- Preface to the first Russian edition
- Preface to the second Russian edition
- Table of Contents
- I. Elements of gasdynamics and the classical theory of shock waves
- II. Thermal radiation and radiant heat exchange in a medium
- III. Thermodynamic properties of gases at high temperatures
- IV. Shock tubes
- V. Absorption and emission of radiation in gases at high temperatures
- VI. Rates of relaxation processes in gases
- VII. Shock wave structure in gases
- VIII. Physical and chemical kinetics in hydrodynamic processes
- IX. Radiative phenomena in shock waves and in strong explosions in air
- X. Thermal waves
- XI. Shock waves in solids
- XII. Some self-similar processes in gasdynamics
- Cited references
- Appendix - Some often used constants, relations between units, and formulas*
- Author Index - Volumes I and II
- Subject Index - Volumes I and II