
- 176 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Thermodynamics
About this book
Indisputably, this is a modern classic of science. Based on a course of lectures delivered by the author at Columbia University, the text is elementary in treatment and remarkable for its clarity and organization. Although it is assumed that the reader is familiar with the fundamental facts of thermometry and calorimetry, no advanced mathematics beyond calculus is assumed.
Partial contents: thermodynamic systems, the first law of thermodynamics (application, adiabatic transformations), the second law of thermodynamics (Carnot cycle, absolute thermodynamic temperature, thermal engines), the entropy (properties of cycles, entropy of a system whose states can be represented on a (V, p) diagram, Clapeyron and Van der Waals equations), thermodynamic potentials (free energy, thermodynamic potential at constant pressure, the phase rule, thermodynamics of the reversible electric cell), gaseous reactions (chemical equilibria in gases, Van't Hoff reaction box, another proof of the equation of gaseous equilibria, principle of Le Chatelier), the thermodynamics of dilute solutions (osmotic pressure, chemical equilibria in solutions, the distribution of a solute between 2 phases vapor pressure, boiling and freezing points), the entropy constant (Nernst's theorem, thermal ionization of a gas, thermionic effect, etc.).
Partial contents: thermodynamic systems, the first law of thermodynamics (application, adiabatic transformations), the second law of thermodynamics (Carnot cycle, absolute thermodynamic temperature, thermal engines), the entropy (properties of cycles, entropy of a system whose states can be represented on a (V, p) diagram, Clapeyron and Van der Waals equations), thermodynamic potentials (free energy, thermodynamic potential at constant pressure, the phase rule, thermodynamics of the reversible electric cell), gaseous reactions (chemical equilibria in gases, Van't Hoff reaction box, another proof of the equation of gaseous equilibria, principle of Le Chatelier), the thermodynamics of dilute solutions (osmotic pressure, chemical equilibria in solutions, the distribution of a solute between 2 phases vapor pressure, boiling and freezing points), the entropy constant (Nernst's theorem, thermal ionization of a gas, thermionic effect, etc.).
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER I
Thermodynamic Systems
1. The state of a system and its transformations
. The state of a system in mechanics is completely specified at a given instant of time if the position and velocity of each mass-point of the system are given. For a system composed of a number N of mass-points, this requires the knowledge of 6N variables.
In thermodynamics a different and much simpler concept of the state of a system is introduced. Indeed, to use the dynamical definition of state would be inconvenient, because all the systems which are dealt with in thermodynamics contain a very large number of mass-points (the atoms or molecules), so that it would be practically impossible to specify the 6N variables. Moreover, it would be unnecessary to do so, because the quantities that are dealt with in thermodynamics are average properties of the system; consequently, a detailed knowledge of the motion of each mass-point would be superfluous.
In order to explain the thermodynamic concept of the state of a system, we shall first discuss a few simple examples.
A system composed of a chemically defined homogeneous fluid. We can make the following measurements on such a system: the temperature t, the volume V, and the pressure p. The temperature can be measured by placing a thermometer in contact with the system for an interval of time sufficient for thermal equilibrium to set in. As is well known, the temperature defined by any special thermometer (for example, a mercury thermometer) depends on the particular properties of the thermometric substance used. For the time being, we shall agree to use the same kind of thermometer for all temperature measurements in order that these may all be comparable.
The geometry of our system is obviously characterized not only by its volume, but also by its shape. However, most thermodynamical properties are largely independent of the shape, and, therefore, the volume is the only geometrical datum that is ordinarily given. It is only in the cases for which the ratio of surface to volume is very large (for example, a finely grained substance) that the surface must also be considered.
For a given amount of the substance contained in the system, the temperature, volume, and pressure are not independent quantities; they are connected by a relationship of the general form:
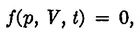
(1)
which is called the equation of state. Its form depends on the special properties of the substance. Any one of the three variables in the above relationship can be expressed as a function of the other two by solving equation (1) with respect to the given variable. Therefore, the state of the system is completely determined by any two of the three quantities, p, V, and t.
It is very often convenient to represent these two quantities graphically in a rectangular system of co-ordinates. For example, we may use a (V, p) representation, plotting V along the abscissae axis and p along the ordinates axis. A point on the (V, p) plane thus defines a state of the system. The points representing states of equal temperature lie on a curve which is called an isothermal.
A system composed of a chemically defined homogeneous solid. In this case, besides the temperature t and volume V, we may introduce the pressures acting in different directions in order to define the state. In most cases, however, the assumption is made that the solid is subjected to an isotropic pressure, so that only one value for the pressure need be considered, as in the case of a fluid.
A system composed of a homogeneous mixture of several chemical compounds. In this case the variables defining the state of the system are not only temperature, volume, and pressure, but also the concentrations of the different chemical compounds composing the mixture.
Nonhomogeneous systems. In order to define the state of a nonhomogeneous system, one must be able to divide it into a number of homogeneous parts. This number may be finite in some cases and infinite in others. The latter possibility, which is only seldom considered in thermodynamics, arises when the properties of the system, or at least of some of its parts, vary continuously from point to point. The state of the system is then defined by giving the mass, the chemical composition, the state of aggregation, the pressure, the volume, and the temperature of each homogeneous part.
It is obvious that these variables are not all independent. Thus, for example, the sum of the amounts of each chemical element present in the different homogeneous parts must be constant and equal to the total amount of that element present in the system. Moreover, the volume, the pressure, and the temperature of each homogeneous part having a given mass and chemical composition are connected by an equation of state.
A system containing moving parts. In almost every system that is dealt with in thermodynamics, one assumes that the different parts of the system either are at rest or are moving so slowly that their kinetic energies may be neglected. If this is not the case, one must also specify the velocities of the various parts of the system in order to define the state of the system completely.
It is evident from what we have said that the knowledge of the thermodynamical state alone is by no means sufficient for the determination of the dynamical state. Studying the thermodynamical state of a homogeneous fluid of given volume at a given temperature (the pressure is then defined by the equation of state), we observe that there is an infinite number of states of molecular motion that correspond to it. With increasing time, the system exists successively in all these dynamical states that correspond to the given thermodynamical state. From this point of view we may say that a thermodynamical state is the ensemble of all the dynamical states through which, as a result of the molecular motion, the system is rapidly passing. This definition of state is rather abstract and not quite unique; therefore, we shall indicate in each particular case what the state variables are.
Particularly important among the Thermodynamical states of a system are the states of equilibrium . These states have the property of not varying so long as the external conditions remain unchanged. Thus, for instance, a gas enclosed in a container of constant volume is in equilibrium when its pressure is constant throughout and its temperature is equal to that of the environment.
Very often we shall have to consider transformations of a system from an initial state to a final state through a continuous succession of intermediate states. If the state of the system can be represented on a (V, p) diagram, such a transformation will be represented by a curve connecting the two points that represent the initial and final states.
A transformation is said to be reversible when the successive states of the transformation differ by infinitesimals from equilibrium states. A reversible transformation can therefore connect only those init...
Table of contents
- DOVER BOOKS ON PHYSICS
- Title Page
- Copyright Page
- Preface
- Table of Contents
- Introduction
- CHAPTER I - Thermodynamic Systems
- CHAPTER II - The First Law of Thermodynamics
- CHAPTER III - The Second Law of Thermodynamics
- CHAPTER IV - The Entropy
- CHAPTER V - Thermodynamic Potentials
- CHAPTER VI - Gaseous Reactions
- CHAPTER VII - The Thermodynamics of Dilute Solutions
- CHAPTER VIII - The Entropy Constant
- Index
- CATALOG OF DOVER BOOKS - Physics
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Thermodynamics by Enrico Fermi in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physics. We have over one million books available in our catalogue for you to explore.