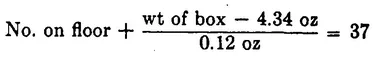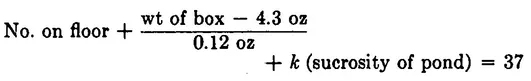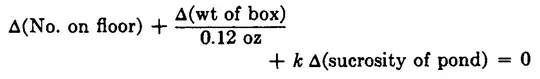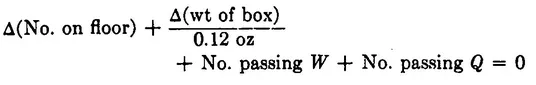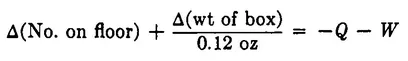![]()
1
Energy Conservation—The First Law of Thermodynamics
What is thermodynamics? Very briefly, it is the study of energy and its transformations. We can also say immediately that all of thermodynamics is contained implicitly within two apparently simple statements called the First and Second Laws of Thermodynamics. If you know anything about these laws, you know that they have to do with energy—the first, explicitly, and the second, implicitly. The First Law says that energy is conserved. That’s all; you don’t get something for nothing. The Second Law says that even within the framework of conservation, you can’t have it just any way you might like it. If you think things are going to be perfect, forget it. The Second Law invokes a quantity called entropy, something that is not part of our experience, so we’ll let it go for a time and consider first the First Law. There is a certain logic in taking up the first things first, and furthermore it allows us to deal with something we all know about, namely, energy.
What is energy? One might expect at this point a nice clear, concise definition. Pick up a chemistry text, a physics text, or a thermodynamics text, and look in the index for “Energy, definition of,” and you find no such entry. You think this may be an oversight; so you turn to the appropriate sections of these books, study them, and find them to be no help at all. Every time they have an opportunity to define energy, they fail to do so. Why the big secret? Or is it presumed you already know? Or is it just obvious?
For the moment, I’m going to be evasive too, but I’ll return to the question. Whatever it is, one thing we know about energy is that it is conserved. That’s just another way of saying that we believe in the First Law of Thermodynamics. Why do we believe in it? Certainly no one has proved it. On the other hand, no one has been able to find anything wrong with it. All we know is that it has always worked in every instance where it has been applied, and we are happy with it simply because it works. Why does it work? We haven’t the faintest idea; it’s just a miracle of nature. The conservation law is a description of how nature works, not an explanation. Fortunately that’s all we need.
Although we do not know why it works, we do know how it works. Any conservation law says that something doesn’t change, and any use of the law just involves accounting. We know there is a fixed amount of something, and we need merely find the various pieces that add up, or account for, the total. To give you an idea of how this is done, I am going to tell a ridiculous story. I’ve stolen the idea of this story from Richard Feynman, Nobel Prize-winning physicist and professor at the California Institute of Technology. His “Lectures on Physics”1 should be studied by every serious student of science and technology.
It is the story of 37 sugar cubes, a small boy, and his mother. To set the scene, I will ask you to imagine the boy’s room at a corner of a house in rural surroundings. The room has two windows, one facing west and the other facing north. For identification, we will call them window W (for west) and window Q (sorry about that). It happens that window W overlooks a small pond. The boy (perhaps his name is Dennis) plays in this room, and his mother looks in from time to time. One day he asks his mother for some blocks to play with. She has no blocks, but she decides that sugar cubes will do. So she gives him 37 sugar cubes and tells him he is not to eat any or he’ll be punished. Each time she returns to the room she counts the sugar cubes lying around, and they total 37; so all is well. But one day she counts and finds only 35. Now Dennis points to an old cigar box he plays with, and his mother starts to open it. But Dennis screams and says, “Don’t open the box”. The mother, of course, realizes she could open the box anyway, but she’s an intelligent, modern mother, and she realizes that this would be a traumatic experience for the boy; so she takes another course.
Later in the day, when she again sees 37 sugar cubes lying about, she weighs the empty box, getting a value of 4.34 oz. She also weighs a sugar cube, getting a value of 0.12 oz. Now the clever lady sets up a formula by which she can check the number of sugar cubes:
This formula works perfectly for quite a time. The left side always totals 37. But one day it does not. Two sugar cubes are missing. As she ponders this problem, she notices that window W is open. She looks out and realizes that the missing sugar cubes could be dissolved in the pond. This taxes her ingenuity, but she was once a nurse and knows how to test the pond for sugar. So she adds a new term to her formula, obtaining
and determines the proportionality constant k by tossing a cube into the pond herself.
This fixes up her formula, and again it works perfectly, accounting always for 37 sugar cubes. As she uses the formula, she begins to realize she could make her work easier if she dealt with changes in the various terms from one checking of the formula to the next. From this point of view the formula can be written as
where the symbol “Δ” means change of. This equation simply says that if sugar cubes are conserved, the sum of all changes in the number of sugar cubes in various places must be zero. This equation too works perfectly for a long period, but one day it fails. The sum comes out not zero, but – 4. Four sugar cubes are missing! This time it doesn’t take mother long to notice that both windows are open and that she has no term in her equation to account for sugar cubes thrown out through window Q. She does not see any sugar cubes on the ground outside, but she does see several squirrels running about. How can she possibly keep track of all that goes on outdoors? The pond was bad enough, but what about squirrels and who knows what else? Her husband, an electrical engineer, solves her problem by building a detection system at each window that counts the sugar cubes as they fly past, so it is no longer necessary to keep track of what happens outdoors. It is only necessary to record what passes through the walls of the room. The mother revises her formula again to reflect the new accounting procedure:
Note that we did not put Δ’s with the two new terms. They need not be thought of as a change in anything. They just represent a number of objects passing a boundary during the interval between checks. In fact, we may as well simplify these terms to read W and Q. We can also transpose them to the other side of the equation; the result is
You see that we are getting more and more technical, and when this happens, technical terms also begin to appear. We may as well introduce several such terms here. Notice that we have narrowed our attention down to the room and to its walls, i.e., to a small region of space. In technical language, we call the room our system, and the walls become its boundary. Everything outside the boundary is called the surroundings. We would very much like to get rid of the surroundings because of their infinite complexity, but we can’t really ignore them. On the other hand, we can make our formula look like it deals only with the system. The last form in which we wrote our formula puts the terms that have to do with changes in the system on the left. On the right we have terms to show what passes out of the system, but they are really there to account for changes in the surroundings. By associating them with the boundary of the system we make the appearance of dealing solely with the system. We treat Q and W as quantities, not as changes in anything, but in fact they are there to account for changes in the surroundings. Any conservation law must somehow include both the system and its surroundings. By insisting that we can account for the surroundings by counting at the system boundary, we are in fact adding something new to the content of a conservation law. It is a bit subtle but becomes obvious once pointed out. We do not expect one of Dennis’ sugar cubes suddenly to disappear from his room on one side of the world and simultaneously to reappear someplace on the other side of the world, even though the other side is part of the surroundings. Why not? No simple statement of a conservation law excludes this. But it just isn’t reasonable; it doesn’t make sense. We’ll leave it at that. The point is that the system-and-its-boundary formula excludes this possibility. We could also exclude it by insisting that conservation exists between a system and its local surroundings, but then we would have to define “local” as any part of the universe with which the system interacts. Then we would find it necessary to define “interacts,” and so on. The beauty of the mathematical system-and-its-boundary formula is that it avoids this chain of verbiage, and this is one of the major advantages of the use of mathematics in the formulation of the laws of science, not that conservation of sugar cubes is a law of science—not yet, at any rate. So let’s return to Dennis, his mother, and the 37 sugar cubes.
All is going well, except that Dennis’ final sugar cube just entered the surroundings. It’s time for a new game, and Dennis’ mother dumps a handful of sugar cubes in his cigar box. This time she doesn’t even count them. Can she still play the game? She certainly can, and she can even delay the start. All it takes is an initial observation and the setting of the window counters to zero. The formula always works, ...