
- 352 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
The Restless Universe
About this book
This highly readable introduction to modern physics was written by a giant of quantum mechanics. Gifted with a rare ability to explain complicated scientific concepts to lay readers, Nobel laureate Max Born presents a step-by-step guide to the understanding of molecules, atoms, subatomic particles, and nuclear physics. Chemical and Engineering News praised Born's narrative as "masterfully discussed . . . easy and delightful," and Philosophy of Science declared that it "should be welcomed by all."
Starting with explanations of molecular motion and the kinetic theory of gases, Born advances to the laws of chance, conduction of heat, molecular weight, relativity, mass and energy, electronic charges, gaseous ions, light waves, light quanta, and spectral lines of gases. Subsequent topics include electron waves, Bohr's theory of the hydrogen atom, wave mechanics, Pauli's exclusion principle, cosmic rays, nuclear structure, and dozens of related subjects. Profusely illustrated with helpful figures and drawings, the text includes an extensive appendix that explains the historical and social significance of developments in modern physics.
Starting with explanations of molecular motion and the kinetic theory of gases, Born advances to the laws of chance, conduction of heat, molecular weight, relativity, mass and energy, electronic charges, gaseous ions, light waves, light quanta, and spectral lines of gases. Subsequent topics include electron waves, Bohr's theory of the hydrogen atom, wave mechanics, Pauli's exclusion principle, cosmic rays, nuclear structure, and dozens of related subjects. Profusely illustrated with helpful figures and drawings, the text includes an extensive appendix that explains the historical and social significance of developments in modern physics.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER IV
The Electronic Structure of the Atom
1. The Discovery of the Positive Charges in the Atom.
NOW we know enough about the electron to be able to study the structure of the atom accurately.
The first question is one which we have already asked more than once, but have not yet answered: where is the positive electricity in the atom?
For, as the whole atom is electrically neutral and it has negative electrons in its outer regions, there must be a positive charge somewhere inside.
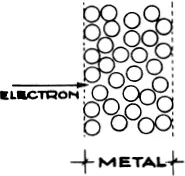
We must therefore penetrate into the interior of the atom. For this purpose we shall find fast particles useful, as they have great momentum. The first systematic investigations of this kind were made by Lenard, using fast cathode rays. As we have already mentioned (p. 94), these will pass through thin layers of metal. In solid bodies the atoms are tightly packed together. The outer shells of electrons of one atom are almost touching those of the next. If an electron flies through with only a small deviation of direction it must have passed through the interior of the atom almost undisturbed (65). This interior must therefore be comparatively empty, in spite of its compact outer layer.
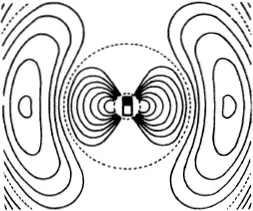
Occasionally, an electron is strongly deviated; we naturally suppose that it has struck some solid obstacle. Lenard was able to explain the bulk of his observations by assuming that in the interior of the atom there are a number of heavy positively charged particles, which he called “dynamids”.
We shall not, however, use these experiments to explain how such conclusions can be drawn, but those of Rutherford instead. Rutherford used heavier projectiles, the α-particles from radioactive substances, which we have already mentioned (p. 102). The advantage of these is that they are not deviated in the slightest by collision with the much lighter electrons; they register only their collisions with particles which are at least about as heavy as they are themselves.
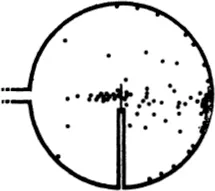
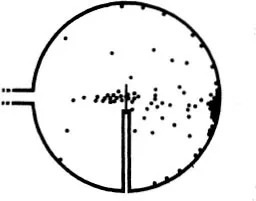
The experiments showed in the first place that most of the α-particles pass through thin metal foil without deviation, but a very minute fraction are noticeably scattered. It follows that in the interior of atoms there must be small heavy particles. These are called atomic nuclei.
We can learn a lot more about the nuclei by accurate observation of the deviated α-particles. For by counting the particles which fly through a bombarded sheet of metal in different directions, we can deduce the laws of interaction of the projectile and the particle struck by it. Direct observation of the path, or at least of its most interesting part, is impossible, for this most interesting part, where the change from one straight line to another takes place, is certainly of atomic dimensions and hence is invisible even in the Wilson chamber.
(66)
(67)
(68)
(69)
We have examples of these collisions in (68) and (69) and Film V. These are drawn according to the laws of ordinary mechanics. This is reasonable enough; for we are dealing with comparatively heavy particles, where, as we know, the quantum effects do not amount to much. We shall come back to this point again (p. 170).
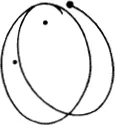
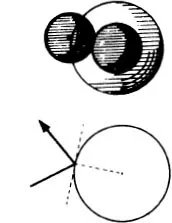
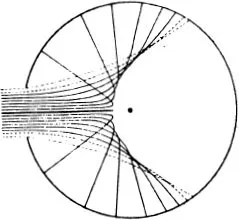
Figure (68) shows the limiting case where the mutual action of the moving particle and the particle at rest is quite trifling until they come within a certain distance of one another, when there is a sudden and large increase in the force. If we think of a sphere described about the stationary particle, whose radius is equal to the distance between the particles at the instant when the repulsive force suddenly rises, the state of affairs is just the same as if very small particles were rebounding elastically from that sphere, like billiard balls (66). In the figure the sphere is shown as a circle. The small particles rebound off it without diminution of velocity, in such a way that their directions after collision make the same angles with the tangent to the circle as they did before collision. We see in (68) that more particles are thrown backwards than forwards. If, however, we carry out the same construction not in the plane but in space, we find that a uniform bundle of flying particles is scattered in all directions.
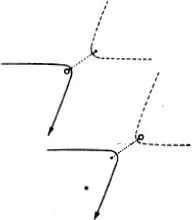
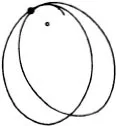
Figure (69) shows the same phenomenon when the mutual action of the moving particle and the obstacle, instead of setting in suddenly when they come into direct contact and then disappearing again, extends to some distance. We have in fact assumed that the repulsion is that which acts between two particles with electric charges of the same sign, the so-called Coulomb’s law, which has just the same form as Newton’s law of gravitation. The force is inversely proportional to the square of the distance; but the force is a repulsion, not an attraction. The paths of the particles are hyperbolas, like the paths of comets, except that with comets the sun lies at the inner focus, whereas with our particles the centre of repulsion lies at the outer focus (67). We see that the directional distribution of the reflected particles is quite different. Every single particle suffers some deviation; but for particles which fly past at a considerable distance the deviation is very trifling. Thus by far the greater part of the particles are practically undeviated.
(69)
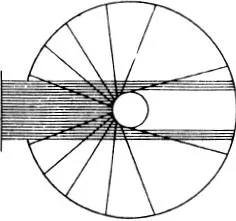
The particles which approach the centre of repulsion closely are scattered vigorously, but by no means uniformly in all directions; many more are scattered forwards than backwards, the fall-off being rapid. This is Rutherford’s law of scattering.
For another law of force we should have another law of scattering.
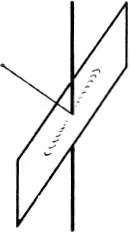
If we let a fine beam of α-rays fall on a thin sheet of metal and count the particles deviated in a particular direction, of course it i...
Table of contents
- Cover
- Title Page
- Copyright Page
- Contents
- List of Plates
- I. The Air And Its Relatives
- II. Electrons and Ions
- III. Waves and Particles
- IV. The Electronic Structure of the Atom
- V. Nuclear Physics
- Postscript
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access The Restless Universe by Max Born in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physics. We have over one million books available in our catalogue for you to explore.