
- 544 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Atomic Physics: 8th Edition
About this book
First published in English in 1935, this classic treatment is well known to students and teachers of physics around the world. Since its original publication, Professor Born (Nobel laureate, 1954) continually updated the book to incorporate new developments in all branches of physics, particularly in the field of elementary particles. For this eighth edition he also wrote a new chapter on the quantum theory of solids.
Contents include:
Kinetic theory of gases
Elementary particles
Spin of the electron and Paul's principle
The nuclear atom
Wave-corpuscles
Atomic structure and spectral lines
Quantum statistics
Molecular structure
Quantum theory of solids
Nuclear physics
Over 40 helpful appendixes, dealing with the mean square deviation, theory of relativity, electron theory, the Compton effect, Hamiltonian theory and action variables, atomic form factor, meson theory, van der Waals forces, and other topics supplement the main text. A bibliography and numerous figures and graphs further enhance the usefulness of Atomic Physics, which retains its value as a broad treatment of basic physics from the special perspective of a towering figure in the field.
Contents include:
Kinetic theory of gases
Elementary particles
Spin of the electron and Paul's principle
The nuclear atom
Wave-corpuscles
Atomic structure and spectral lines
Quantum statistics
Molecular structure
Quantum theory of solids
Nuclear physics
Over 40 helpful appendixes, dealing with the mean square deviation, theory of relativity, electron theory, the Compton effect, Hamiltonian theory and action variables, atomic form factor, meson theory, van der Waals forces, and other topics supplement the main text. A bibliography and numerous figures and graphs further enhance the usefulness of Atomic Physics, which retains its value as a broad treatment of basic physics from the special perspective of a towering figure in the field.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
ATOMIC PHYSICS
CHAPTER I
Kinetic Theory of Gases
1. Atomic Theory in Chemistry.
In present-day physics, the concepts of energy and matter are connected in the most intimate way with atomic theory. We therefore begin with a brief discussion of the rise of ideas relating to the atom. The source of these ideas, we know, is chemistry. They suggest themselves almost inevitably when we try to interpret the simple regularities which are at once disclosed when the masses of the substances transformed in chemical reactions are determined quantitatively with the balance. It is established in the first place, that in a reaction the total weight remains unchanged. Secondly, it is found that substances combine only in fixed simple proportions by weight, so that a definite weight of one substance can only enter into reaction with definite weights of a second substance; and the ratio of these weights is independent of the external conditions, such as, for example, the proportion by weight in which the two substances may have been mixed. These regularities are expressed in the language of the chemist in the laws of constant and of multiple proportions (Proust, 1799; Dalton, 1808), e.g.:
1 gm. hydrogen combines with 8 gm. oxygen to form 9 gm. water,
1 gm. hydrogen combines with 35·5 gm. chlorine to form 36·5 gm. hydrogen chloride.
An example of the law of multiple proportions is given by the compounds of nitrogen and oxygen: 7 gm. nitrogen combine with

In the case of gases, simple laws hold not only for the weights of the reacting substances but also for their volumes (Gay-Lussac, 1808). Thus (at constant pressure)
2 vols. hydrogen combine with 1 vol. oxygen to form 2 vols. water vapour,
1 vol. hydrogen combines with 1 vol. chlorine to form 2 vols. hydrogen chloride.
The numbers expressing the ratios by volume are those which appear in the corresponding chemical formulæ. In the preceding examples, for instance, we have
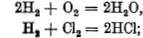
and
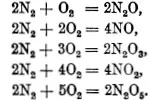
These facts may be interpreted as follows, as was done by Avogadro: every gas consists of a great number of particles, its atoms or molecules; and equal volumes of all gases, at the same temperature and pressure, contain the same number of molecules.
The significance of this principle in relation to the laws of chemical reactions may be illustrated from the above examples. The fact that two volumes of hydrogen combine with one volume of oxygen to form two volumes of water vapour is (Avogadro, 1811) equivalent to the statement that two molecules of hydrogen combine with one molecule of oxygen to form two molecules of water. Similarly, the combination of one part by weight of hydrogen with eight parts by weight of oxygen to form nine parts by weight of water means that a molecule of oxygen must be eight times, and two molecules of water nine times, as heavy as two molecules of hydrogen.
We are thus led to the concepts of molecular weight and atomic weight. These are, respectively, the weights of a molecule and of an atom of the substance in question. They are not measured in grammes, but with reference to a standard (ideal) gas, the atomic weight of which is put equal to 1; and it has been agreed to define this, not so that H = 1, but so that C = 12; this convention has turned out very lucky (because of the existence of the heavy isotope of hydrogen, p. 68), We shall denote the molecular weight measured in this way by μ.
That quantity of a substance, the weight of which is μ gm., is called a mole (even when the substance is not capable of existence in the chemical sense). A mole of oxygen atoms therefore weighs 16 gm., but a mole of oxygen molecules weighs 32 gm. From this definition of the mole it follows that the quantity “1 mole” always contains the same number of molecules. This number of molecules per mole plays a great part in the kinetic theory of gases. The number of molecules per cubic centimetre from which this quantity can be derived was first measured by Loschmidt in 1865. However, at the present time the convention is to refer to the number of molecules per cubic centimetre as Loschmidt’s number and the number per mole as Avogadro’s number. We denote the latter by N0; its value is (see p. 23)
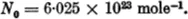
As a consequence of Avogadro’s law, 1 mole of any gas at a given pressure p and a given temperature T always occupies the same volume; for a pressure of 760 mm. of mercury and a temperature of 0° C. the volume is 22·4 litres.
We add here an explanation of a number of symbols which will be employed below. If m is the mass of a molecule in grammes, then μ = N0m; in particular, for atomic hydrogen (μ almost exactly = 1), N0mH = 1. If further n is the number of molecules in the unit of volume, N that in the volume V, and if v is the number of moles in the volume V, then we have vN0 = nV = N. Finally, we denote by ρ = nm the density of the gas, and by vs = 1/ρ its specific volume.
2. Fundamental Assumptions of the Kinetic Theory of Gases.
After these prefatory remarks on the atomic theory in chemistry, we now proceed to the kinetic theory of gases (Herapath, 1821; Waterston, 1843; Krönig, 1856). Considering the enormous number of particles in unit volume of a gas, it would of course be a perfectly hopeless undertaking to attempt to describe the state of the gas by specifying the position and velocity of every individual particle. As in all phenomena of matter in bulk, we must here have recourse to statistics. But the statistics now to be used is of a somewhat different kind from that which we are acquainted with in ordinary life. There, the statistical method consists in recording a large number of events which have occurred, and in drawing conclusions from the numerical data so obtained. Thus, mortality statistics answers the question of how much more probable it is that a man will die at 60 than at 20 years of age; for this purpose we count the number of ca...
Table of contents
- Cover
- Title Page
- Copyright Page
- Dedication
- Preface
- Preface to Eight Edition
- Contents
- Chapter I Kinetic Theoey of Gases
- Chapter II Elementary Particles
- Chapter III The Nuclear Atom
- Chapter IV Wave-Corpuscles
- Chapter V Atomic Structure and Spectral Lines
- Chapter VI Spin of The Electron and Pauli’s Principle
- Chapter VII Quantum Statistics
- Chapter VIII Molecular Structure
- Chapter IX Quantum Theory of Solids
- Chapter X Nuclear Physics
- Appendices
- Bibliography
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Atomic Physics: 8th Edition by Max Born in PDF and/or ePUB format, as well as other popular books in Ciencias físicas & Física. We have over one million books available in our catalogue for you to explore.