
eBook - ePub
Kinetic Theory of Gases
Walter Kauzmann
This is a test
- 272 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Kinetic Theory of Gases
Walter Kauzmann
Book details
Book preview
Table of contents
Citations
About This Book
Written by a former Princeton University professor specializing in the thermal properties of matter, this monograph and text was designed for first-year students of physical chemistry who require further details of kinetic theory. The treatment focuses chiefly on the molecular basis of important thermodynamic properties of gases, including pressure, temperature, and thermal energy. Extended and often quite elementary presentations of abstract basic concepts offer students the opportunity to grasp the essentials of modern physical chemistry. Thermodynamic and molecular theories of heat are developed side by side, so that thermodynamic theory is constantly illuminated and enhanced by molecular theory.
Topics include equations of state of gases and empirical gases, the molecular explanation of the equations of state, and the molecular theory of the thermal energy and heat capacity of a gas. Additional subjects include the distribution of molecular velocities in a gas as well as molecular collisions and the transport properties of gases. Numerous exercises, many of them partially worked out, help students internalize concepts and illustrate practical uses and special applications. End-of-chapter problems offer further reinforcement.
Topics include equations of state of gases and empirical gases, the molecular explanation of the equations of state, and the molecular theory of the thermal energy and heat capacity of a gas. Additional subjects include the distribution of molecular velocities in a gas as well as molecular collisions and the transport properties of gases. Numerous exercises, many of them partially worked out, help students internalize concepts and illustrate practical uses and special applications. End-of-chapter problems offer further reinforcement.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Kinetic Theory of Gases an online PDF/ePUB?
Yes, you can access Kinetic Theory of Gases by Walter Kauzmann in PDF and/or ePUB format, as well as other popular books in Sciences physiques & Chimie physique et théorique. We have over one million books available in our catalogue for you to explore.
Information
Topic
Sciences physiquesSubtopic
Chimie physique et théoriqueChapter 1
EQUATIONS OF STATE OF CASES;
EMPIRICAL RESULTS
1-1 The “primary properties,” mass, volume, pressure, and temperature
Gases are substances which completely fill any container into which they are introduced. Let us suppose that standing before the student on his desk as he reads this chapter is a flask containing some gaseous substance such as air. The air in this flask has a great many properties—color, odor, density, velocity, viscosity, thermal conductivity, electrical conductivity, magnetic susceptibility, dielectric constant, chemical composition, and so on. In this chapter, however, we shall be concerned with four particularly important properties of a gaseous substance: mass, volume, pressure, and temperature. Because they are so important and are so frequently measured by chemists we shall call them the “primary properties.” Before considering their relationship to each other, let us be sure that we understand what we mean by these four properties.
The properties in question are expressible as numbers, and can be best defined if we describe how one goes about determining these numbers. (This way of looking at scientific concepts is almost always the safest way of being sure that one knows exactly what a concept means, and deciding the circumstances under which the concept loses or changes its significance.)
The mass of a gas in a vessel can be determined by weighing the vessel when it contains the gas, and then weighing the vessel again after the gas has been pumped out. This procedure is straightforward, and although it is possible to imagine some subtle complications regarding the concept of mass, these complications play no role in physical chemistry, at least not under ordinary circumstances.
The volume of a gas can be most easily determined by weighing the water required to fill the vessel containing the gas, since the volume of a unit mass of water between 0°C and 100°C is very accurately known. The procedure is simple in principle, but a complication arises if the gas and the vessel are at an elevated temperature, where the mass of water per unit of volume may not be so well known. This complication can be overcome, of course, by using other liquids whose densities are known at the desired temperature. Furthermore, if the vessel has a simple geometry (a cube, sphere, cylinder, etc.), we may measure its dimensions and the volume may then be determined by means of a suitable geometric formula.
A more awkward complication arises in determining the mass of a given volume or the volume of a given mass of a gas when the gas is not in a container—say when it is flowing out of a nozzle or around the wing of an airplane. We shall avoid complications of this kind for the present, however, by considering only gases in closed vessels. On the other hand, we shall be very much concerned with vessels whose volumes can be altered—particularly with vessels in which one wall can be moved—for instance, a gas contained in a piston chamber or a gas under compression by a liquid in a gas burette (Fig. 1-1). Clearly, each position of the piston or of the liquid corresponds to a volume which can be determined in principle in the manner described above.
The concept of pressure, although apparently simple in principle, is in practice subject to a good many qualifications. It may be measured by means of a manometer, with which one observes the height of a column of an inert liquid, such as mercury, that is balanced by the force exerted by the gas on the liquid surface. (This measurement will be described in more detail below, in Section 1-3.1). If a gas and its container are at rest, and if they are located in a region free of gravitational fields, then the pressure that the gas exerts on the container walls is the same at all points on all of the walls. Under these conditions one can speak of the pressure on the walls as “the pressure of the gas,” and this pressure can be regarded as a property of the entire gas sample. On the other hand, if the system is located in a gravitational field, the pressure on the walls will not be uniform. A gas at the earth’s surface exerts a force on the bottom of its container which is greater by the weight of the gas than the force on the top of the container. Strictly speaking, such a gas sample as a whole does not have “a pressure.” If the box is small, however, and if the gas is not highly compressed, these pressure differences will be negligible so that one is in the habit of speaking of “the pressure” of the gas despite the fact that it cannot be precisely defined under these circumstances.
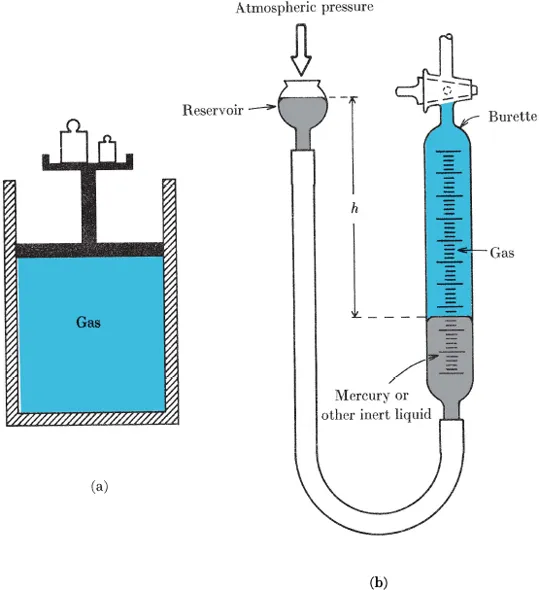
FIG. 1-1 Devices for observing the behavior of the volume of a gas when the pressure on the gas is changed, (a) Conceptual piston-chamber device. The pressure may be changed by changing the weights on the platform. (b) Gas burette. Pressure may be changed by moving the reservoir up and down and the volume may be read off from the graduations on the burette.
EXERCISE One liter of air at room temperature and at one atmosphere pressure (= about 1 kg/cm2 at the earth’s surface) has a mass of about 1 g. What is the percentage difference in pressure between the top and bottom of a column which is 1 m in height, filled with air at one atmosphere and located at the earth’s surface?
A more awkward complication arises if a gas moves relative to the walls of its container—as will be the case if the gas is swirling about or if one of the walls (say the piston in Fig. 1-1) is in motion. The pressure may then be quite different on different walls, and again it is not possible to speak of “the pressure” of the whole gas sample. This complication will...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Editor’s Foreword
- Preface
- Contents
- Introduction
- Chapter 1: Equations of state of gases; empirical gases
- Chapter 2: The molecular explanation of the equations of state
- Chapter 3: The molecular theory of the thermal energy and heat capacity of a gas
- Chapter 4: The distribution of molecular velocities in a gas
- Chapter 5: Molecular collisions and the transport properties of gases
- Index of Symbols
- Index of Subjects
- Back Cover
Citation styles for Kinetic Theory of Gases
APA 6 Citation
Kauzmann, W. (2013). Kinetic Theory of Gases ([edition unavailable]). Dover Publications. Retrieved from https://www.perlego.com/book/112850/kinetic-theory-of-gases-pdf (Original work published 2013)
Chicago Citation
Kauzmann, Walter. (2013) 2013. Kinetic Theory of Gases. [Edition unavailable]. Dover Publications. https://www.perlego.com/book/112850/kinetic-theory-of-gases-pdf.
Harvard Citation
Kauzmann, W. (2013) Kinetic Theory of Gases. [edition unavailable]. Dover Publications. Available at: https://www.perlego.com/book/112850/kinetic-theory-of-gases-pdf (Accessed: 14 October 2022).
MLA 7 Citation
Kauzmann, Walter. Kinetic Theory of Gases. [edition unavailable]. Dover Publications, 2013. Web. 14 Oct. 2022.