![]()
Chapter 1
Function Follows Form
Tension and tightness dissolve a mile into the run. My breathing evens out. My heart beats an easy cadence. My calm, detached mind enjoys the ride without interfering. I float along the bike path, legs limber, feet brushing the ground lightly, each step propelling the next, my movements effortless, integrated, exhilarating. I am—at least for the moment and at least in my own mind—a fluid, graceful machine.
Efficient human movements require resilience, the physiological ability to store and return energy. Without resilient tendons, the threads of tissue that connect muscles to bones, human movement would be jerky and wasteful. Tendons specialize in resilience, but they also must be strong and stiff—able to handle heavy loads and resist changes in length. Strength, stiffness, and resilience arise from the unique structure of collagen, the main component of tendons. Other tissues require different properties. Arteries, the vessels that carry blood away from the heart, share with tendons the property of resilience, but must also be extensible—able to stretch without snapping. Their extensibility comes from a molecule called elastin. Just as the physical structures of muscles and bones influence their function, the chemical structures of collagen and elastin mold their functional properties.
When we exercise, we move bones, changing their relationship to each other. The elbow joint begins a biceps curl in an extended position, with bones of the forearm and upper arm aligned. As the biceps muscle shortens, the elbow joint flexes, bringing the forearm toward the shoulder. More complicated athletic actions—such as triple axels, baseball swings, and pole vaults—may require hundreds of separate bone movements. But all these maneuvers ultimately reduce to a series of movements of one bone with respect to another.
Muscles and tendons partner to move bones during exercise—muscles generate force, tendons transmit the force to bones. Although muscles may attract more attention at the beach and more ink in the fitness magazines, tendons are equally essential to successful movements. Intermediaries between muscles and bones, tendons link muscular force to physical action. Tendons attach each muscle to at least two different bones. For example, tendons join one end of the biceps muscle to the shoulder blade and the other end to the forearm near the elbow. Because of these connections, the forearm moves toward the shoulder when the biceps shortens.
Throughout history, humans have exploited the remarkable properties of tendons. The armies of Dionysius the Elder likely used crossbows reinforced by animal tendons as early as 399 BC. Writing in the first century AD, Hero of Alexandria described the use of tendons in catapult torsion springs. And for centuries, people have fashioned animal tendons into bowstrings. An effective bowstring resists breaking when pulled back, transmits force without large changes in its length, and stores energy when stretched. In other words, bowstrings are strong, stiff, and resilient, so the qualities that make tendons effective transmitters of force from muscle to bone also make tendons good bowstrings. As I said earlier, the properties of tendons come from the molecular makeup of collagen. Collagen, which accounts for 80 percent of the dry weight of tendons, belongs to a class of molecules that will engage our attention throughout this book: proteins.1
Proteins are tiny tools, devices, and machines, analogous to the gadgets that pack a typical kitchen. Just as kitchen tools—pans, knives, blenders, bread machines—perform various tasks, proteins play varied roles in our bodies. Some proteins regulate cell processes; others form cell structures. Some proteins produce mechanical force; others catalyze chemical reactions. Some proteins move molecules in and out of cells; others drag molecules from one place to another inside cells. Almost all proteins interact with other molecules—including other proteins—in the course of their duties, just as a knife and cutting board collaborate to dice vegetables. And just as a blender can be used for countless purposes, many proteins perform multiple functions.
Form determines the effectiveness of both kitchen implements and proteins. We grasp knives, forks, and spoons by shafts that have the same shape because they serve the same role—they are handles. Other parts of each utensil reflect a specific function: knife blades cut, fork tines spear, and spoon bowls scoop. Within each category of utensil, structures vary to match tasks. Butter spreaders, steak knives, and vegetable cleavers modify the basic knife design to accomplish specialized functions. As with utensils, the three-dimensional structure of proteins dictates their capabilities. Some proteins, the teaspoons of the protein world, have simple structures. Other proteins are more like bread machines, complex assemblages with myriad moving parts. Different proteins share common structural motifs with widely useful functions in the same way that different utensils share the same handle design. At the same time, many proteins contain unique structural regions necessary for distinct functions, much like blades, bowls, and tines of utensils. In short, proteins flaunt an extravagant array of shapes. Collagen assembles into ropelike strands. Other proteins are oval, helical, planar, globular, filamentous, and just about every other shape you can imagine.
Manufacturers fashion kitchen utensils using a variety of materials and processes. In contrast, all proteins share the same building blocks. To make proteins, cells link simple molecules called amino acids into chains that fold into innumerable shapes. Envision a protein’s amino acids as beads on a necklace. Then imagine a necklace comprising twenty different kinds of beads, equivalent to the number of different amino acids. We can fashion 8,000 different necklaces or proteins by linking just three beads or amino acids (20 × 20 × 20 = 203 = 8,000). Necklaces of 200 beads—the number of amino acids in a typical protein—can come in 20200 or 10260 (10 followed by 259 zeros) combinations. Thus a stunning variety of necklaces—and proteins—is possible. Now, imagine that some beads are magnetic, so they attract or repel other beads, twisting the necklace into a complex shape. Meanwhile, large beads constrain the necklace, preventing it from bending certain ways. The sequence of beads determines exactly how the necklace folds; if two nearby beads attract each other, the necklace will twist differently than if two distant beads attract. The shape of our imaginary necklace—how it coils and contorts—depends on the properties and sequence of its beads.
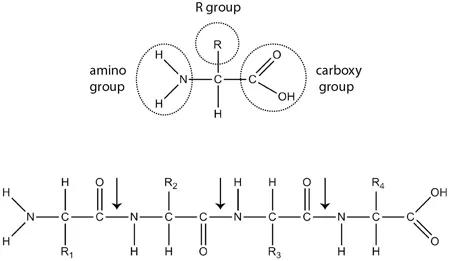
Likewise, protein structure arises from the properties and sequence of amino acid chains. Amino acids consist of a central carbon atom connected to four groups (Figure 1.1). Three of these groups—the hydrogen atom, the carboxylic acid group, and the amino group—are invariant across all amino acids. The fourth group—the side group—varies in shape, electrical charge, chemical reactivity, and solubility in water. A protein’s linear sequence of amino acids determines how it folds. For example, side groups with positive charges repulse other positively charged groups but attract groups with negative charges. Similarly, water-soluble amino acids often line protein surfaces, facing outward toward the surrounding water. Although factors such as temperature and acidity also influence protein shape, amino acid sequence plays the leading role. The nearly limitless permutations of amino acid sequence make possible the dazzling diversity of protein forms.2
1.1. Amino acid chemistry. Above: Chemical structure of an amino acid. Below: Four amino acids joined into a peptide. Arrows point to peptide bonds.
Collagen’s amino acid sequence displays a distinctive pattern: many repeats of a three-amino-acid motif, represented as Gly-X-Y. Each triplet starts with the small amino acid glycine (Gly). Though the remaining positions (X and Y) can be any amino acid, the bulky amino acid proline (or the related molecule hydroxyproline) often fills them. How does collagen’s repeating motif relate to the unique properties of tendons? We will answer this question first with respect to strength and stiffness, and then turn to resilience.
Many modern athletes can sympathize with Achilles, the mythical Greek hero. Achilles’s mother held his heel when she dipped him in the River Styx, making his entire body except that heel invincible. Achilles eventually died, as the story goes, when a poisoned arrow lodged in his susceptible heel. Though today’s athletes rarely need to dodge poison arrows, vulnerable heels still plague us. Many sports require movements that seriously stress the aptly named Achilles tendon, which connects calf muscles to the calcaneus bone of the heel.3
Forces on the Achilles peak during sprinting, reaching almost ten times body weight, about 1,500 pounds in an adult. Yet, though it is thicker than any other tendon in our body, the Achilles is surprisingly thin, with a diameter between 6 and 7 millimeters (mm), about the width of a standard pencil. Consequently, the cross-sectional area of the Achilles is about a hundred-fold less than that of the calf muscle it serves. The Achilles’s slim profile affords it access to the compact ankle joint. A skinny Achilles also lightens the foot, saving energy because weight at the end of a limb is especially burdensome to move. High forces acting on a thin tendon means that the Achilles must be particularly strong. Imagine a Toyota Prius, which weighs about 3,000 pounds, suspended from two pencil-thin Achilles tendons, and you get a sense of the Achilles tendon’s strength. Though they occasionally fail, Achilles tendons can withstand high forces before they break.4
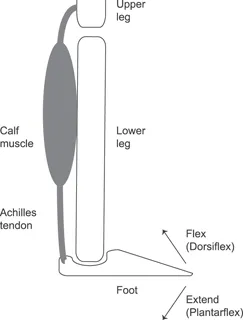
The Achilles must not only be strong, but also stiff—it must not stretch too much when force is applied. To see why, test the mobility of your ankle. Extend the ankle by contracting your calf, pushing your toes downward toward the sole of your foot and away from the shinbone (Figure 1.2). Then, flex the ankle by pulling your toes upwards toward the shinbone, and feel the Achilles tendon stretch.5 Your foot follows a similar pattern during running, hitting the ground in a relaxed, somewhat extended position, then flexing as weight rolls onto it. The ankle’s modest range of motion matches the properties of the Achilles. About 250 mm long, the Achilles tendon is a bit longer than a standard pencil. When the ankle flexes, the Achilles extends by about 6 percent. Thus, despite bearing large forces during running, the Achilles tendon stretches only a small amount. Stiff tendons effectively transmit muscular force from the calf to the heel. When tendons lose their stiffness, muscular force stretches the tendon rather than moving the bone, impairing force transfer.
1.2. Diagram of lower leg and foot.
Let’s return to collagen’s structure to see where tendons get their strength and stiffness. Chemical bonds hold together the individual amino acids in collagen and other proteins. Two amino acids attach when the carboxylic acid group of one amino acid reacts with the amino group of another, forming a covalent bond (Figure 1.1). The atoms that participate in covalent bonds share electrons, gluing the atoms together strongly. Hence, protein chains are tough to break. Still, when exposed to forces like those on the Achilles, a single collagen strand would snap like a line of spider silk stretched across the Indy 500 finish line. To achieve adequate strength, many collagen molecules must cooperate.
Each collagen molecule contains about a thousand amino acids with many Gly-X-Y repeats. The tiny side groups of glycine, each a single hydrogen atom, form notches between the larger side groups in the X and Y positions. Three collagen threads click together at these indentations, forming a tightly wound triple helix.6 Amino acids at the X and Y positions further stabilize the helix by forging hydrogen bonds between separate strands. Hydrogen bonds form due to the attraction between partial negative and positive changes. Although weak compared to covalent bonds, many hydrogen bonds combine to brace the helix. Weaving three strands together enhances strength. Notches and hydrogen bonds prevent strands from sliding alongside each other, contributing to the stiffness of the triple helix and making it tougher to stretch.7
The collagen triple helix resembles rope. Both draw strength from the braiding of individual strands. Both require strands to be held together to prevent slippage when a force is applied. And both are strong in tension when their ends are pulled apart, but not as effective in opposing compression when their ends are pushed together.
Though stronger than individual collagen molecules, triple helices are still much too weak to transduce muscular force. Each helix is only 1.3 nanometers (nm) thick and 300 nm long. Nanometers are definitely small—one nanometer is 0.00000004 inches—but it can be hard to picture lengths that short, so let’s pause for a moment and try to envision what these measurements mean. Nanometers are a thousand-fold smaller than micrometers (μm), which in turn are a thousand-fold smaller than millimeters (mm). Thus nanometers are six orders of magnitude smaller than the smallest intervals on a metric ruler. To think of it another way, millimeters eclipse nanometers to the same extent that kilometers eclipse millimeters. Any way we slice it, many tiny collagen triple helices must be packed together to form a 6 mm-by-250 mm Achilles tendon.
Cells build tendons by secreting collagen triple helices into the extracellular matrix, a mixture of proteins and sugars outside cells. At this site, hundreds of triple helices group into fibrils, held tightly together by covalent bonds between amino acids at the X or Y position of Gly-X-Y triplets. In turn, fibrils aggregate into tendons with thousands of robustly attached collagen molecules running in parallel. In summary, the strength of tendons comes not from the individual talents of collagen molecules, but rather from their knack for collaboration.
Despite collagen’s strength, painful injuries to the Achilles tendon commonly afflict recreational athletes. The worst type of Achilles damage, a partial or complete rupture of the tendon, sometimes triggers a sharp popping sensation that victims compare to a gunshot. Nobody wants to be hobbled by an Achilles injury, so runners learn to respect and nurture their Achilles tendons. Before and after workouts, you’ll find us pushing against a wall, one leg extended back. We first straighten the extended leg to stretch our calves and then slowly bend at the knee to gently tug on our Achilles tendons. The idea is to stretch the muscles and tendons of the lower leg, decreasing their stiffness, relieving some of the stress on the Achilles.
Whether our rituals improve performance or reduce injury is a matter of some debate. Stretching does decrease the stiffness and increase the length of muscle-tendon units, which could decrease injury risk. But most controlled studies of stretching fail to demonstrate benefits. Even worse, muscle strength often decreases directly after static stretching—holding positions for fifteen or more seconds—impairing performance in activities that demand strength. One reason for these strength declines is that reduced stiffness delays transmission of force from muscles to bone. Other factors, such as impaired activation of muscles by nerves, also contribute. Regardless of the mechanisms, static stretching prior to exercise, especially exercise that requires powerful contractions, may be counterproductive. Some research suggests that dynamic stretching, which uses sports-specific movements rather than held poses, may be more beneficial than static stretching.8
In contrast to its negative effects on strength, stretching improves range of motion and flexibility, thereby enhancing activities such as dancing where those qualities matter. Lowering stiffness also tends to improve resilience, which ...