
Retrosynthetic Analysis and Synthesis of Natural Products 1
Synthetic Methods and Applications
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Retrosynthetic Analysis and Synthesis of Natural Products 1
Synthetic Methods and Applications
About This Book
For chemists, attempting to mimic nature by synthesizing complex natural products from raw material is a challenge that is fraught with pitfalls. To tackle this unique but potentially rewarding task, researchers can rely on well-established reactions and methods of practice, or apply their own synthesis methods to verify their potential. Whatever the goal and its complexity, there are multiple ways of achieving it. We must now establish a strategic and effective plan that requires the minimum number of steps, but lends itself to widespread use. This book is structured around the study of a dozen target products (butyrolactone, macrolide, indole compound, cyclobutanic terpene, spiro- and polycyclic derivatives, etc.). For each product, the different disconnections are presented and the associated syntheses are analyzed step by step. The key reactions are described explicitly, followed by diagrams showing the range of impact of certain transformations. This set of data alone is conducive to understanding syntheses and indulging in this difficult, but worthwhile activity.
Frequently asked questions
Information
1
Total Synthesis: Some Elements to Contemplate
1.1. Total synthesis – why and for what purpose?
| Industry | Annual tonnage as products | Factor E | Annual tonnage as waste | Number of people of steps |
| Petrochemical | 106–108 t | 0.1 | 107 t | separation |
| Heavy chemistry | 105–106 t | <1–5 | 5.106 t | 1–2 |
| Fine chemistry | 100–104 t | 5–50 | 5.105 t | 3–4 |
| Pharmaceutical | 10–1000 t | 25–> 100 | 105 t | > 6 |
1.2. The different approaches
- – direct application of this method;
- – interest in the compound’s properties, or its unique and even fascinating structure.
- – By hemisynthesis: one of the most well-known cases is that of taxol, a diterpene initially isolated from the bark of Taxus baccata and known for its highly cytotoxic properties. To collect sufficient quantities, Françoise Guéritte and Pierre Potier’s team at the ICSN in Gif-sur-Yvette succeeded in isolating 10-deacetylbaccatin III from yew tree needles, a precursor with the same taxane skeleton; it was possible to regioselectively graft the appropriate side chain onto the hydroxy group at the C-13 position of the taxane skeleton [DEN 88, DAN 96].
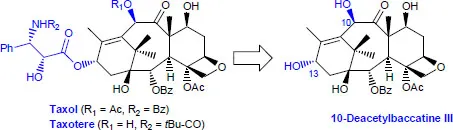 Figure 1.2. Taxol and Taxotere® from 10-deacetylbaccatin III
Figure 1.2. Taxol and Taxotere® from 10-deacetylbaccatin III - – According to a chiron approach: it is a question of benefiting from molecular platforms and carrying out various selective transformations. Stephen Hanessian is one of the researchers who has made the most out of this concept [HAN 12, BRI 17]. In the synthesis of dihydromevinolin, L-glutamic acid was used as a molecular block to prepare the lactone synthon with two stereogenic centers, which after several steps, gives rise to the key precursor of cycloaddition according to Diels–Alder [HAN 87, HAN 90].
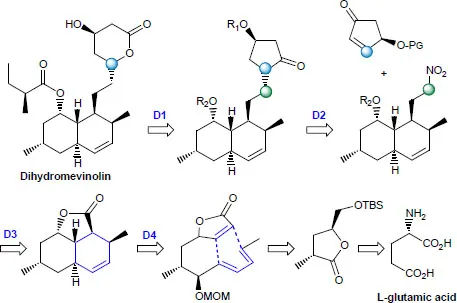 Figure 1.3. Dihydromevinolin from L-glutamic acid
Figure 1.3. Dihydromevinolin from L-glutamic acid
- D1: ring enlargement (Baeyer–Villiger reaction).
- D2: introd...
Table of contents
- Cover
- Table of Contents
- Preface
- 1 Total Synthesis: Some Elements to Contemplate
- 2 Squamostolide
- 3 Rubrenolide
- 4 Bipinnatin J
- 5 Tubingensin B
- 6 Polygonatine A
- 7 (+)-Intricatetraol
- 8 Enigmazole A
- 9 Biyouyanagin A
- 10 Elatol
- 11 Thiomarinol H
- 12 Oblongolides A and C
- List of Abbreviations
- Index
- End User License Agreement