
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Introduction to Materials Chemistry
About this book
This textbook introduces the reader to the elementary chemistry on which materials science depends by discussing the different classes of materials and their applications. It shows the reader how different types of materials are produced, why they possess specific properties, and how they are used in technology. Each chapter contains study questions to enable discussions and consolidation of the acquired knowledge.
The new edition of this textbook is completely revised and updated to reflect the significant expansion of the field of materials chemistry over the last years, covering now also topics such as graphene, nanotubes, light emitting diodes, extreme photolithography, biomedical materials, and metal organic frameworks.
From the reviews of the first edition:
"This book is not only informative and comprehensive for a novice reader, but also a valuable resource for a scientist and/or an industrialist for new and novel challenges." (Materials and Manufacturing Process, June 2009)
"Allcock provides a clear path by first describing basic chemical principles, then distinguishing between the various major materials groups, and finally enriching the student by offering a variety of special examples." (CHOICE, April 2009)
"Proceeding logically from the basics to materials in advanced technology, it covers the fundamentals of materials chemistry, including principles of materials synthesis and materials characterization methods." (Internationale Fachzeitschrift Metall, January 2009)
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part I
Background to Materials Chemistry
1
What Is Materials Chemistry?
A. Different Types of Materials
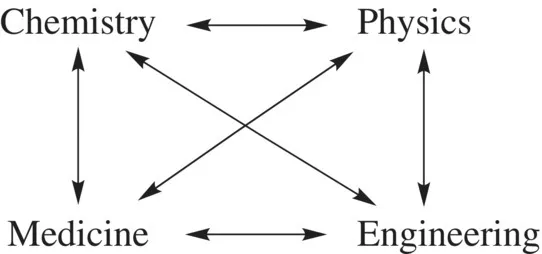
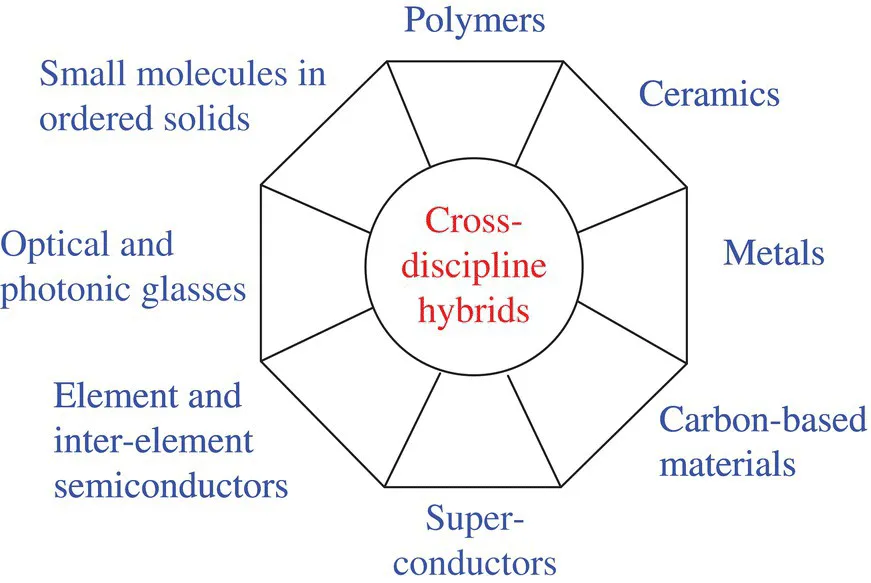
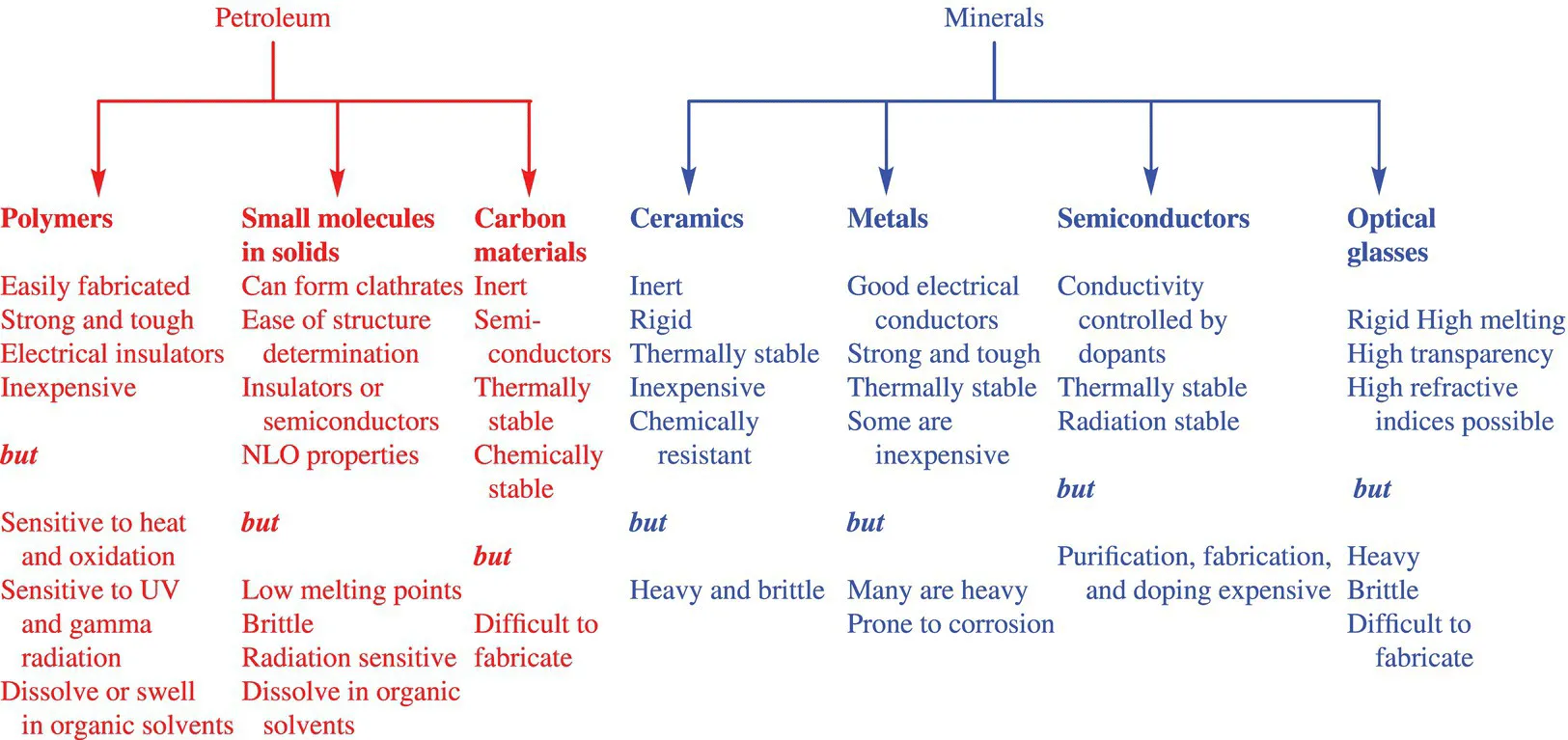
Table of contents
- Cover
- Table of Contents
- Preface to the Second Edition
- Part I: Background to Materials Chemistry
- Part II: Different Types of Materials
- Part III: Materials in Advanced Technology
- Appendix Terminology
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app