
Modern Supercritical Fluid Chromatography
Carbon Dioxide Containing Mobile Phases
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Modern Supercritical Fluid Chromatography
Carbon Dioxide Containing Mobile Phases
About This Book
Explains why modern supercritical fluid chromatography (SFC) is the leading "green" analytical and purification separations technology.
Modern supercritical fluid chromatography (SFC) is the leading method used to analyze and purify chiral and achiral chemical compounds, many of which are pharmaceuticals, pharmaceutical candidates, and natural products including cannabis-related compounds. This book covers current SFC instrumentation as it relates to greater robustness, better reproducibility, and increased analytical sensitivity.
Modern Supercritical Fluid Chromatography: Carbon Dioxide Containing Mobile Phases covers the history, instrumentation, method development and applications of SFC. The authors provided readers with an overview of analytical and preparative SFC equipment, stationary phases, and mobile phase choices. Topics covered include: Milestones of Supercritical Fluid Chromatography; Physical Properties of Supercritical Fluids; Instrumentation for SFC; Detection in SFC; Achiral SFC Method Development; Chiral SFC Method Development; and Preparative Scale SFC. The book also includes highlights of modern applications of SFC in the final chapters—namely pharmaceuticals, consumer products, foods, polymers, petroleum-related mixtures, and cannabis—and discusses the future of SFC.
- Provides a clear explanation of the physical and chemical properties of supercritical fluids, which gives the reader a better understanding of the basis for improved performance in SFC compared to HPLC and GC
- Describes the advantages of SFC as a green alternative to HPLC and GC for the analysis of both polar, water-soluble, and non-polar analytes
- Details both achiral and chiral SFC method development, including modifiers, additives, the impact of temperature and pressure, and stationary phase choices
- Details why SFC is the premier modern preparative chromatographic technique used to purify components of mixtures for subsequent uses, both from performance and economic perspectives
- Covers numerous detectors, with an emphasis on SFC-MS, SFC-UV, and SFC-ELSD (evaporative light scattering detection)
- Describes the application of SFC to numerous high-value application areas
Modern Supercritical Fluid Chromatography: Carbon Dioxide Containing Mobile Phases will be of great interest to professionals, students, and professors involved in analytical, bioanalytical, separations science, medicinal, petroleum, and environmental chemistries. It will also appeal to pharmaceutical scientists, natural-product scientists, food and consumer-products scientists, chemical engineers, and managers in these areas.
Frequently asked questions
Information
1
Historical Development of SFC
1.1 Physical Properties of Supercritical Fluids
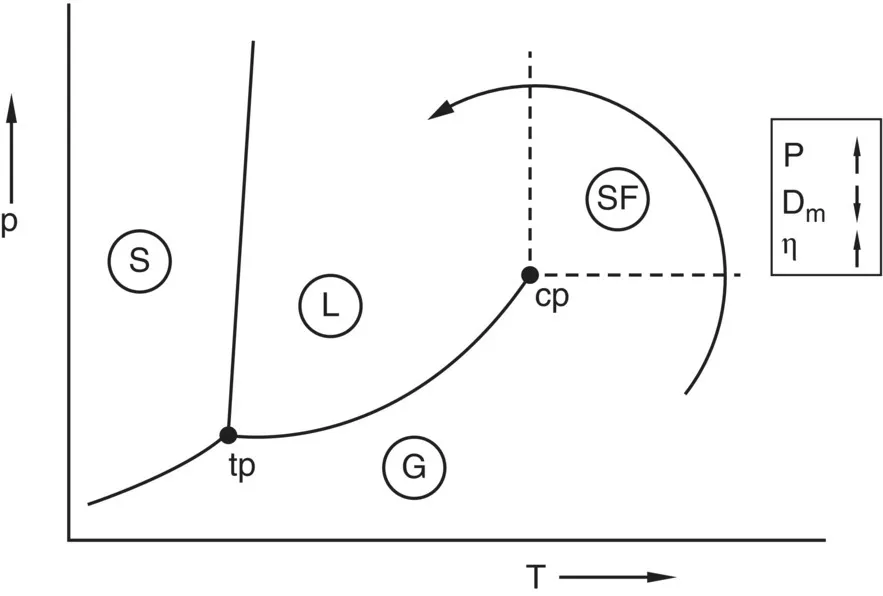
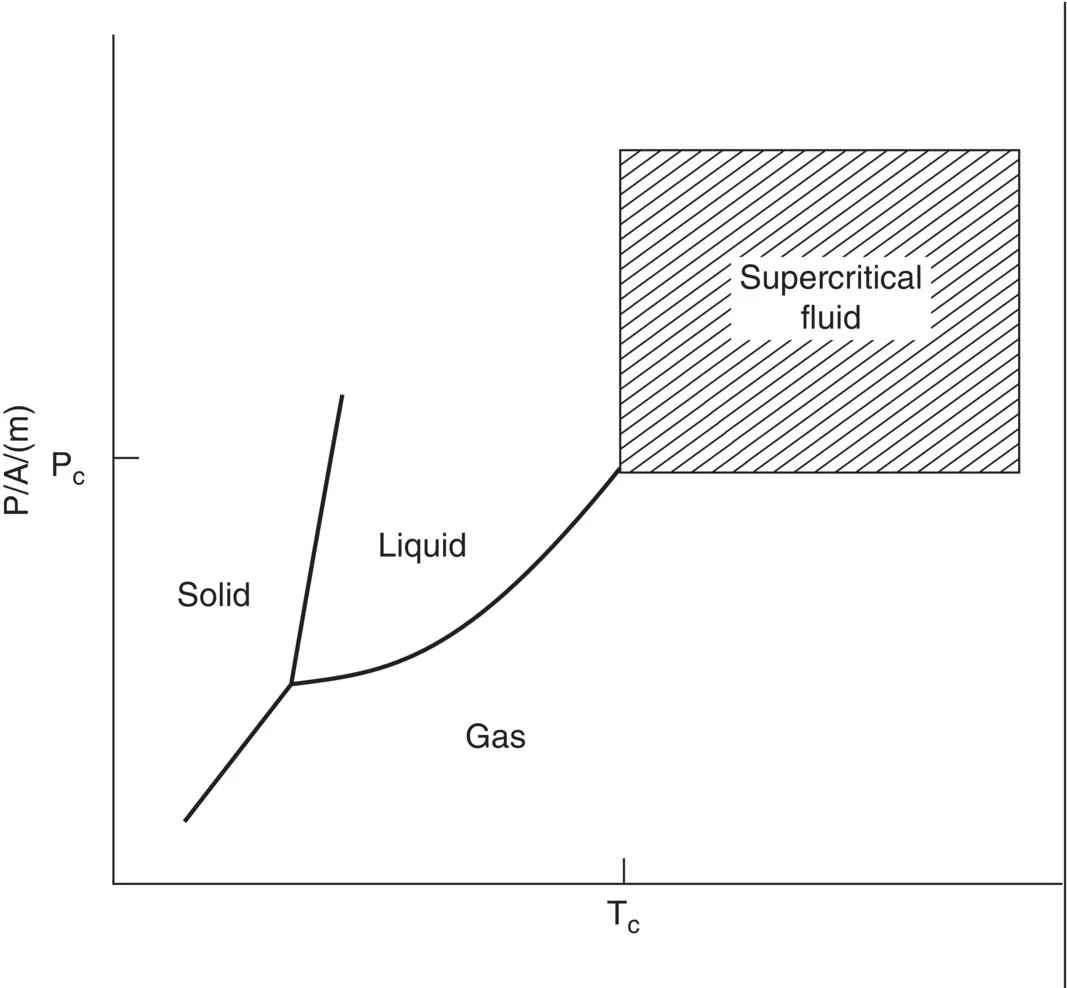
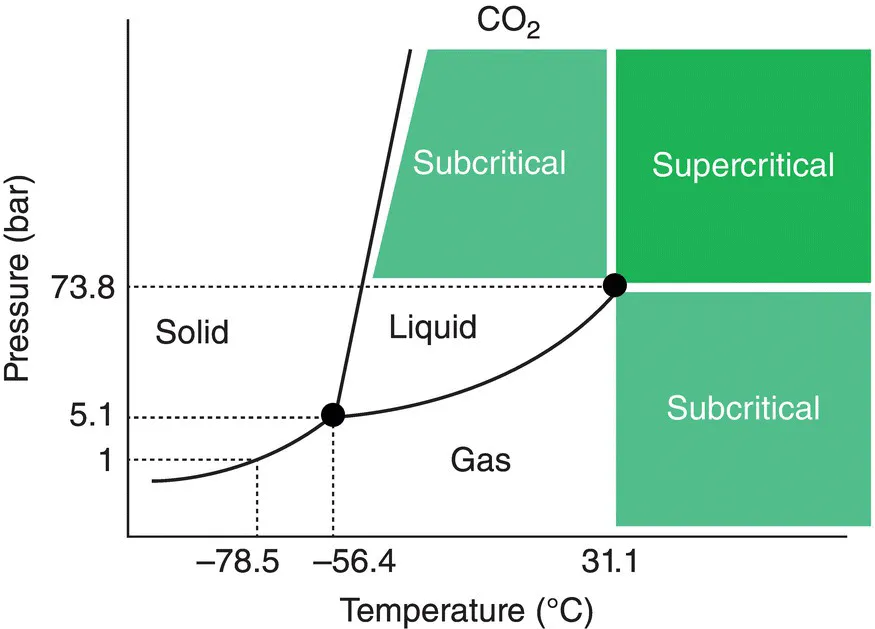
Table of contents
- Cover
- Table of Contents
- Chemical Analysis
- Preface
- 1 Historical Development of SFC
- 2 Carbon Dioxide as the Mobile Phase
- 3 Instrumentation for Analytical Scale Packed Column SFC
- 4 Detection in Packed Column SFC
- 5 Chiral Analytical Scale SFC – Method Development, Stationary Phases, and Mobile Phases
- 6 Achiral Analytical Scale SFC – Method Development, Stationary Phases, and Mobile Phases
- 7 Instrumentation for Preparative Scale Packed Column SFC
- 8 Preparative Achiral and Chiral SFC – Method Development, Stationary Phases, and Mobile Phases
- 9 Impact and Promise of SFC in the Pharmaceutical Industry
- 10 Impact of SFC in the Petroleum Industry
- 11 Selected SFC Applications in the Food, Polymer, and Personal Care Industries
- 12 Analysis of Cannabis Products by Supercritical Fluid Chromatography
- 13 The Future of SFC
- Index
- Chemical Analysis
- End User License Agreement