![]()
CHAPTER 1
Bilayer Electrolytes for Low Temperature and Intermediate Temperature Solid Oxide Fuel Cells – A Review
Alireza Pesaran , Abhishek Jaiswal and Eric D. Wachsman*
Maryland Energy Innovation Institute, University of Maryland, College Park, MD 20742, USA,
*E-mail:
[email protected] The commercialisation of solid oxide fuel cell (SOFC) technology would benefit dramatically by a reduction of the operating temperature to a lower range (500–650 °C). Unfortunately, the ionic conductivity of YSZ and electrode performance decrease significantly at low temperatures resulting in low power density SOFCs. Doped ceria materials have ionic conductivity orders of magnitude higher than YSZ and have been extensively explored as an alternative electrolyte material. However, doped ceria reduces under fuel conditions at the anode side resulting in internal leakage current. This work is primarily focused on reviewing the recent developments of the concept of a bilayer electrolyte SOFC where ceria is the main electrolyte and the second electrolyte serves to block the leakage current. A thorough survey of works in the literature reveals that bismuth oxide/ceria bilayer electrolyte SOFCs yield higher power density compared to zirconia/ceria bilayer electrolyte SOFCS mainly due to the much higher ionic conductivity of stabilised bismuth oxide compositions compared to YSZ. A proper ceria/bismuth oxide thickness ratio is of great importance and hence needs to be tuned carefully. In addition, bilayer electrolytes can serve other functions in SOFC structures such as a diffusion barrier layer between the electrolyte and adjacent electrodes or a fast oxide ion conductor to promote catalytic activity toward oxygen reduction.
1.1 Introduction
Fuel cell technology holds the potential to change the way power is generated, transmitted and utilised in our increasingly energy dependent lifestyles. State-of-the-art solid oxide fuel cells (SOFCs) operate at high temperatures (750–950 °C). Lower operating temperatures would significantly improve the economics of power generation using SOFCs. The aim of this chapter is to evaluate component materials for bilayer electrolyte-based SOFCs that could work efficiently at temperatures below 650 °C.
A fuel cell is an electrochemical device that converts the chemical energy of a fuel and an oxidant into electrical energy. The conversion is direct without the need for intermediate conversion into heat and mechanical energy, as in the case of conventional turbine/generator systems and is not limited by the Carnot cycle with up to 50% achievable electrical efficiency. 1 As fuel cells do not involve any combustion process, there is no formation of pollutants such as NO x , SO x , hydrocarbons and particulates. Also, fuel cells have limited moving parts (blower) and hence do not generate any noise pollution and require minimal maintenance. Due to these advantages over other energy generators, fuel cells are desired in stationary as well as in mobile applications. Among all of the different types of fuel cells, proton exchange membrane fuel cells (PEMFCs), molten carbonate fuel cells (MCFCs) and SOFCs are considered to be the most advanced and closest to wide-scale commercialisation. However, significant technological and engineering challenges remain. When running on hydrocarbon fuels in addition to external reforming, PEMFCs also require CO removal from the fuel feed as they are susceptible to CO poisoning, which results in lower conversion efficiencies. On the other hand, SOFCs run at high temperatures and ideally can internally reform any hydrocarbon fuel with high efficiencies without the need of expensive catalysts. In addition, the high-quality waste heat from the SOFCs can be utilised in cogeneration to further improve the overall efficiency – up to 85% – of the system. 2
State-of-the art SOFCs operate at temperatures between 750 and 950 °C using yttria-stabilised zirconia (YSZ) as the electrolyte material, La(Sr)MnO3-YSZ composite as the cathode material and Ni-YSZ ceramic metal (cermet) composite as the anode material. However, the high operating temperatures of SOFCs put considerable limitations on the choice of materials for the various components and also on the lifetime of the cell. Over the years, research in industry and academia has resulted in the development of materials and fabrication techniques such that the cell components are able to perform well under extreme conditions, to withstand thermal mismatch, to be microstructurally stable and to counter interactions between adjoining components. However, this has led to high material and fabrication costs, making SOFCs less competitive with existing power generation technologies. Decreasing the operating temperatures will considerably improve the economics of power generation using SOFCs by enabling the use of cheap ferritic stainless steel alloys as the interconnect material instead of expensive alloys or ceramics, cheaper balance of plant and insulation along with increased lifetime. 3 Lower operating temperatures will also result in faster start-up that is critical in certain applications. All these factors have led to a global drive towards reducing the operating temperature of SOFCs from 750 to 950 °C to low to intermediate temperatures of 500–750 °C. However, efficient operation at lower temperatures will require new electrolyte materials with higher conductivity and new electrode materials with better catalytic activity at lower temperatures. For the purpose of further discussion, the operating temperature of SOFCs is defined as low temperature (500–650 °C), intermediate temperature (650–800 °C) and high temperature (800–950 °C).
1.2 Material Review
1.2.1 Electrolyte
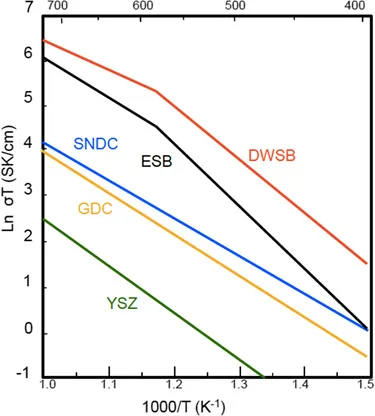
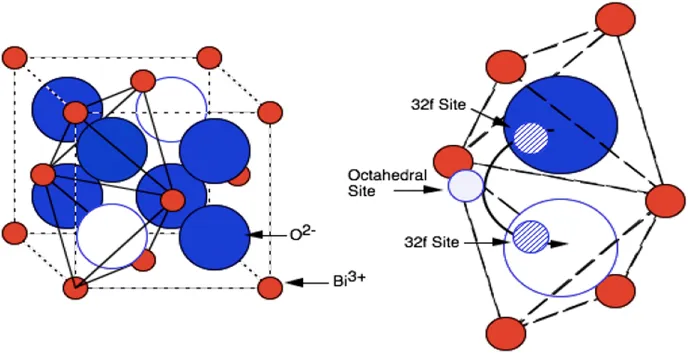
Oxide-ion conducting solid electrolytes are the backbone of SOFCs, allowing selective transport of oxide ions for electrochemical oxidation of fuel to generate electrical power. The electrolyte materials for SOFCs are oxides having a fluorite or perovskite structure because they are larger, loose-packed structures that have the ability to accept a wide range of dopants. Oxide-ion conductivity of common solid-oxide electrolytes is shown in Figure 1.1. 3 The ionic conduction is possibly due to the presence of oxide-ion deficiency in the solid electrolyte, which typically is introduced by doping the host electrolyte with lower valent cations. The fluorite type unit cell is shown in Figure 1.2. CeO2 and ThO2 exhibit a cubic fluorite structure from room temperature up to their melting points. The open fluorite structure offers the possibility of achieving an unusually wide range of solid solutions with alkaline-earth and rare-earth oxides such as CaO and Gd2O3. Generally, when the cation size of the host and the guest are almost equal, the solid solution is easily formed. In the case of ZrO2 and Bi2O3, the high-temperature cubic fluorite structure can be stabilised at lower temperatures by forming a solid solution and hence, the terms stabilised zirconia and stabilised bismuth oxide are used. The electrolyte in the fuel cell is exposed to both the oxidant and the fuel atmospheres, and hence, it should be thermodynamically stable under oxidising and reducing conditions. To avoid the generation of electronic conductivity, the electrolyte should have a large band gap and the dopants introduced into the lattice should not exhibit multiple oxidation states. As the oxygen oxide ion conduction is a thermally activated process, the performance of the electrolyte improves with temperature. However, there are limits in terms of operating temperatures due to material compatibility issues with other components and operational viability over extended periods of time. Reduced operating temperatures will allow the choice of a wider range of materials and significantly improve the economics of power generation using SOFCs. However, operation of SOFCs at intermediate to low temperatures requires better performance electrolyte and electrode materials.
Figure 1.1 Conductivity of oxide-ion conducting solid electrolytes. Reproduced from ref. 3 with permission from AAAS, Copyright 2011.
Figure 1.2 Fluorite structure of δ-Bi2O3 and an oxide-...