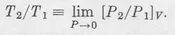![]()
1
Introduction
Thermodynamics is a notably abstract science with innumerable concrete applications. It unites extreme generality with extreme reliability to a degree unsurpassed by any other science. The magnificent generality of thermodynamics arises from the abstractness of its fundamental concepts: stripped insofar as possible of everything referring to specific things and events, such concepts are fitted for the interpretation of relations of the most diverse varieties. The extraordinary reliability of thermodynamics arises because, while calling on only a minimal array of axiomatic postulates, it cunningly contrives to discuss material phenomena without making any assumption whatever about the constitution (atomic or otherwise) of matter.
Thermodynamics treats of systems—parts of the world that definite boundaries separate conceptually (and often, with a good degree of approximation, physically) from the rest of the world. The condition or state of such a system is regarded as thermodynamically defined as soon as values have been established for a small set of measurable parameters.1 These parameters are so chosen that any particular state of the system will be fully and accurately reproduced whenever the defining parameters take on the set of values descriptive of that state. Temperature, pressure, volume, and expressions of concentration (e.g., mole fractions) are the parameters most used by chemists, and temperature in particular is distinctive of thermodynamic analyses generally. But not every state of every system can be characterized by a single well-defined temperature (and pressure), and from this circumstance arises two major restrictions on the applicability of classical thermodynamics. First, given the (Max-wellian) distribution of molecular velocities, a single molecule, or even a small group of molecules, does not have a definite temperature. Only to macroscopic systems will a temperature be assignable, and only to such systems will thermodynamics be applicable. Second, even a macroscopic system will manifest local inhomogeneities of temperature (and pressure) while it is undergoing rapid change. An entire macroscopic system will be characterizable by a unique temperature (and pressure) only when that system stands in an unchanging state of equilibrium—or in a quasi-static state only infinitesimally different from a true equilibrium state. And it is with such states alone that classical thermodynamics concerns itself.
1 For this and all other bibliographical notes indicated by consecutive superscript numbers, see Appendix I on p. 140.
Focused on the equilibrium states of macroscopic systems, classical thermodynamics tells us nothing about the paths by which different states may be connected, and nothing about the rates at which the paths may be traversed and the states attained. Thermodynamics is then, if you please, rather more the science of the possible in principle than the science of the attainable in practice. What thermodynamics finds impossible we cannot hope to achieve, and we are spared the investment of effort in a vain endeavor. But to achieve in practice what thermodynamics finds possible in principle may still require an immense endeavor. For example, thermodynamic calculations show that, under pressures of 30,000-100,000 atmospheres, diamond should be formed from graphite at temperatures of 1000-3000°K. However attempts to achieve this conversion were for long totally unsuccessful. Having confidence in thermodynamics, one attributes these failures to slowness of the reaction, and one perseveres in the endeavor to achieve in practice what thermodynamics indicates as possible in principle. And in 1954 the synthesis of diamond was at last achieved—through the discovery of an effective catalyst, and the construction of equipment competent to maintain the required conditions for hours rather than seconds.2
TEMPERATURE
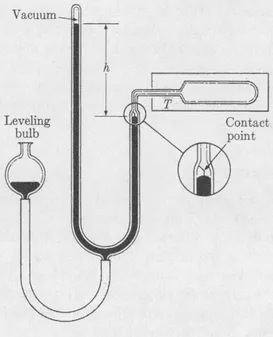
The theoretical term “temperature” first acquires empirical relevance only when we learn how temperature is to be determined. Let it now be specified that temperature is to be measured with a constant-volume gas thermometer, shown schematically in Fig. 1. When the gas in the sealed bulb has come to equilibrium at the temperature (T) prevailing in the enclosure, by appropriate manipulation of the leveling bulb, we bring the mercury meniscus always to the indicated fiducial mark. Having thus assured constancy of the gas volume (V), we obtain the pressure (P) by measurement of the height h. To establish what we mean by temperature, we have then only to write, for the measurements made at any two different temperatures:
where the subscript v underlines the constant-volume requirement. The temperature ratios determined from this formula prove unhappily variable with the identity and pressure of the gas present in the bulb. But whatever the gas used, this variability disappears when we repeatedly determine the pressure ratio with progressively diminished amounts of gas in the bulb, and then extrapolate to the limit P → 0. That is, with any pure gaseous species we obtain the same temperature ratio from the limiting pressure ratio:
To pass from the temperature ratios so defined to numerical values for individual temperatures, we need only adopt a convention that assigns some particular numerical magnitude to the temperature of some one standard reference point. By international agreement,3 we assign 273.16 as the temperature at the “triple point” at which ice, water, and water vapor coexist in equilibrium.a Letting the subscript tp signify the triple point...