
- 238 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Methods of Thermodynamics
About this book
Since there is no shortage of excellent general books on elementary thermodynamics, this book takes a different approach, focusing attention on the problem areas of understanding of concept and especially on the overwhelming but usually hidden role of "constraints" in thermodynamics, as well as on the lucid exposition of the significance, construction, and use (in the case of arbitrary systems) of the thermodynamic potential. It will be especially useful as an auxiliary text to be used along with any standard treatment.
Unlike some texts, Methods of Thermodynamics does not use statistical mechanics as a crutch to explain the subject. In the author's view, the student should learn to use the method of themodynamics in all its power, applying it to any problem it may help solve. As the author states: "In view of the high level of confidence which we place in thermodynamics, what is known thermodynamically is often considered to be known once and for all…by restricting oneself initially to purely thermodynamic arguments, one can know what he does know before entering domains where conclusions are less certain."
Major chapter headings in this volume include: mathematical apparatus, the first law of thermodynamics, the second law and entropy, ideal substances, some useful formulas, internal equilibrium, and the extremal properties of the entropy, thermodynamic potentials, phase equilibria in simple systems, osmotic systems, systems which may perform surface work, systems in gravitational and centrifugal fields, elastic systems, stability, and third law. 1965 edition.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
IV
The Second Law and Entropy
1. General Remarks
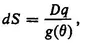

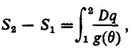
2. Need for an Additional Extensive Function of State
Table of contents
- DOVER BOOKS ON PHYSICS
- Title Page
- Copyright Page
- Dedication
- Preface
- Table of Contents
- I - Some General Concepts
- II - Mathematical Apparatus
- III - The First Law of Thermodynamics
- IV - The Second Law and Entropy
- V - Ideal Substances
- VI - Some Useful Formulas
- VII - Internal Equilibrium and the Extremal Properties of the Entropy
- VIII - Thermodynamic Potentials
- IX - Phase Equilibria in Simple Systems
- X - Osmotic Systems
- XI - Systems Which May Perform Surface Work
- XII - Systems in Gravitational and Centrifugal Fields
- XIII - Elastic Systems
- XIV - Stability
- XV - The Third Law
- Index
- A CATALOG OF SELECTED DOVER BOOKS IN SCIENCE AND MATHEMATICS
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app