
eBook - ePub
Chemical Reaction Engineering
A Computer-Aided Approach
- 252 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Chemical Reaction Engineering
A Computer-Aided Approach
About this book
This book illustrates how models of chemical reactors are built up in a systematic manner, step by step. The authors also outline how the numerical solution algorithms for reactor models are selected, as well as how computer codes are written for numerical performance, with a focus on MATLAB and Fortran. Examples solved in MATLAB and simulations performed in Fortran are included for demonstration purposes.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Introduction
A chemical reactor is a piece of equipment in which the chemical transformation of raw material, the reactants, into products takes place. The chemical reactor is only one part of the entire chemical process, and typically it is preceded in the process by separation units where the raw material is purified. Similarly, the chemical reactor is seldom able to produce pure products from the raw material, as some raw materials remain unreacted and by-products are formed. Therefore, the desired products are separated from the undesired ones in a separation unit after the chemical reactor, as illustrated in the principal flow sheet presented in Figure 1.1.
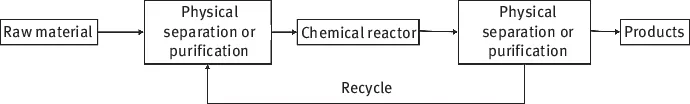
Figure 1.1: Principal flowsheet of a chemical process.
The unreacted reactant molecules are recirculated back into the chemical reactor to maintain a good economy in the utilization of raw material. Thus, the process stages described earlier represent key steps in any chemical process: preparation of the raw material for the chemical transformation, the chemical transformation itself and the separation of valuable reaction products from unreacted raw material and by-products. Several sophisticated separation techniques are at our disposal. The dominating one is distillation, but extraction, absorption, adsorption and crystallization are also commonly used. Development, description and modelling of separation processes are large subfields of chemical engineering, but they are not included in this book.
Our discussion shows that the performance of a chemical reactor is of crucial importance: the better – that is more efficiently and selectively – the chemical reactor is operated, the higher the output of the overall process. A major part of the total cost of a chemical process is usually caused by the separation operations; therefore, the general goal is to perform the reaction as well as possible in the chemical reactors to minimize the costs of separation operations and to achieve a global optimization of the process.
This book deals with the modelling and simulation of chemical reactors, which is at the core of any industrial process designed for chemical transformations. The basis of understanding it lies in the physical–chemical fundamentals, that is, reaction stoichiometry, kinetics and thermodynamics (Chapter 2). The book covers homogeneous reactors where only one phase – gas or liquid – is present (Chapter 3), heterogeneously catalysed systems (catalytic two- and three-phase reactors, Chapters 4 and 5) as well as gas–liquid systems (Chapter 6). Finally, the chemical engineer cannot limit himself to well-known systems, where all the kinetic, thermodynamic and transport parameters are known a priori. In most cases, experiments in laboratory as well as bench and pilot scales are needed. Therefore, the last chapters (7 and 8) are devoted to experiments on laboratory scale and to estimation of parameters from experimental data. The crucial steps of modelling and simulation are illustrated in Figure 1.2.
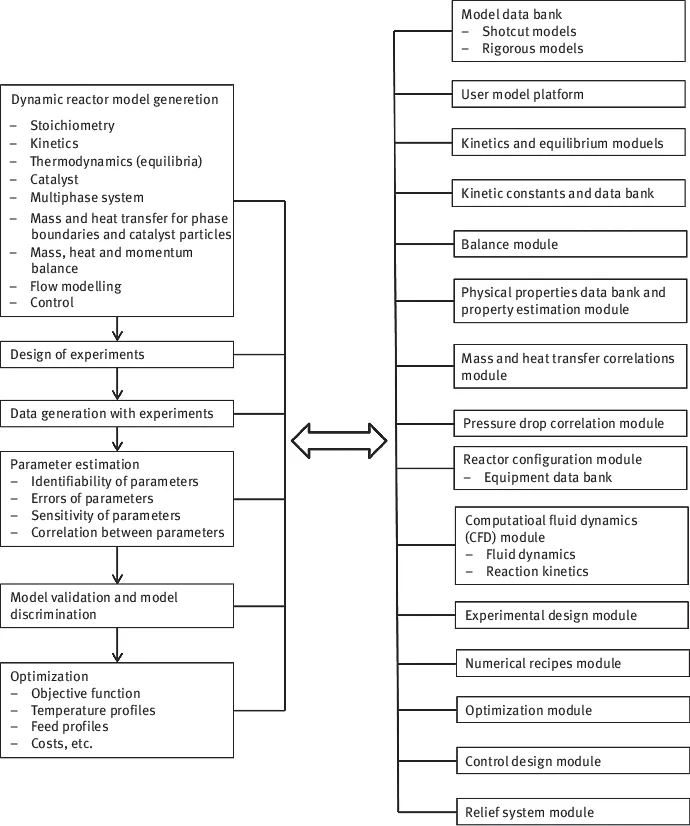
Figure 1.2: Steps of modelling and simulation work (after Tirronen and Salmi (2003)).
Efficient and robust numerical algorithms and computer software play a central role in the successful solution of reactor models. The enormous development of computing speed during the last decades has made the dreams of yesterday a reality: what theoreticians scribbled on paper in the past can be computed exactly today. Real numerical computing of processes enables a more precise, economical and environmentally sustainable process design. We try to provide the reader with a systematic approach and a guided journey from reactor modelling through numerical methods and computer implementation to the final calculation of the reactor performance. Have a nice trip!
2 Kinetics in reaction engineering
Modelling of chemical processes in general and chemical reactors in particular is based on stoichiometry, thermodynamics and kinetics. Reaction stoichiometry tells us the relative amounts of molecules interacting in a chemical process, the quantities of reactants needed for a chemical reaction and the number of product molecules formed. Thermodynamics determines the ultimate conversion limit of a reactant in a chemical reaction; de facto thermodynamic calculations provide the equilibrium composition of a reacting mixture. Chemical kinetics qualitatively and quantitatively explains the velocities, rates of chemical processes, that is, how fast the equilibrium composition is approached in a real chemical reactor.
2.1 Stoichiometry of multiple reactions
The basic c...
Table of contents
- Title Page
- Copyright
- Contents
- Preface
- Nomenclature
- 1 Introduction
- 2 Kinetics in reaction engineering
- 3 Modelling of homogeneous systems
- 4 Modelling of fixed beds and fluidized beds
- 5 Modelling of three-phase systems
- 6 Modelling of gas–liquid systems
- 7 Equipment and models for laboratory experiments
- 8 Parameter estimation in reaction engineering
- Bibliography
- Exercises
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Chemical Reaction Engineering by Tapio Salmi,Johan Wärnå,José Rafael Hernández Carucci,César A. de Araújo Filho in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.