![]()
In cross-referencing the chapters that follow, it became clear that a number of chemical concepts kept re-occurring. So for the consumer tourist, we believe that coming to grips with some of these is well worthwhile. We start by introducing the language of chemicals and their social life. Topics follow fast and furious: nomenclature, phases, solubility, hard and soft acids and bases, chemical speciation, chemical accountancy, chemical activity and free radicals.
The meaning of the word ‘chemistry’ has changed many times over the centuries. In the 3rd century of our common era, it referred to the fraudulent practice of imitating precious metals and stones (Greek khemeia). By the Middle Ages (~410–1453 CE), this practice had evolved into the quest for a substance that would actually transmute base metals into gold, and into the study of matter associated with this process, known as alchemy (from Arabic alkimia). In the 16th century, following the redirection of alchemy towards medicine by Paracelsus, the word came to mean the making of medicines. A dealer in medicinal drugs became a chemist. With Boyle’s critique of alchemical theory in his The Sceptical Chymist of 1661, ‘chemistry’, came to mean also the study of matter in a scientific manner.
Whereas sciences such as astronomy, biology, geology and physics continued to study the natural world, chemistry quickly went out to change it. While there are industrial offshoots from physical, biological and geological sciences, that is not their dominant theme.
Chemistry is involved in every aspect of our lives. In this book, we show how it keeps us safe. We see it in the laundry and the kitchen. Chemistry improves our sporting ability, makes eating safe and fun, provides cosmetics and keeps us safe in the sun. It helps us to improve our gardens and allows us to swim in our pools without getting infections. Chemistry provides plastics and metals to make all sorts of great consumer products, giving us a terrific range of fabrics and easy care clothes. We use chemistry when painting our house, building our homes, running our cars more efficiently and sustainably. It also provides the medicines to keep us healthy and living longer, productive lives.
Admittedly, it is not always positive news and there have been serious mistakes along the way. We do not avoid discussing these and hope your better understanding of the chemistry involved (by reading this book) will reduce them in the future.
Because of its dependence on raw materials and its provision of materials that are essential to other industries, the chemical industry plays a major part in world trade and a significant part in world politics. The various environmental debates have made chemistry even more central to political science.
SOCIAL AND CHEMICAL INTERACTIONS
If you were to define the social structure of a community, you would start by categorising the social interactions. Starting with the strong interactions, you find that they are fewer in number than the weak ones. A (nuclear) family unit has strong interactions, in the sense that the members of a family interact over long periods of time and the individuals influence each other mutually to a large degree. Families are generally relatively small units (2–12 members) and, initially at least, are highly localised. The next step up gives a choice of categories. For example, circles of close family, friends or the people at work. Then there is the club or the church group. The larger the group, the weaker the interactions. The categories are arbitrary, but some are more obvious and useful than others. They are never clear-cut and there are always questions at the edges. Is Uncle Joe, three times removed, family or close friend? Anyone who has made out a wedding invitation list knows the problem!
With chemical bonding, the same problem arises. With extreme examples (i.e. the ones always chosen to illustrate the point) everything is clear-cut. An isolated gaseous molecule of hydrogen chloride is like a couple on their honeymoon: a unique, unperturbed bond between a hydrogen and chlorine atom, oblivious to all other interaction. But when the molecule dissolves in water, the honeymoon is over and all the interactions change.
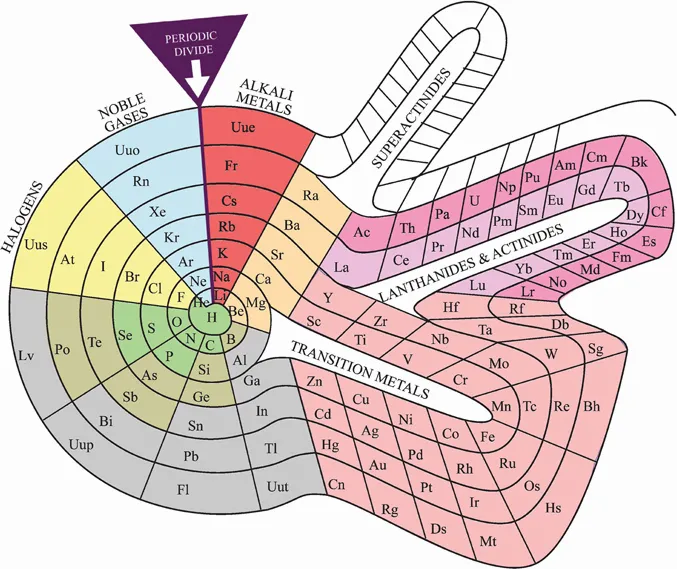
At last count, there are 118 elements known, but systematic chemistry deals with the family life of the 90-plus individual elements that have lifetimes long enough to allow them to be studied and used. The most basic categorisation in chemistry is the periodic table of the chemical elements. It is an icon of chemistry, credited to the Russian chemist Dmitri Mendeleev (1834–1907). The version shown in Fig. 1.1 is an alternative form of the table.
The chemical elements, in order of increasing relative atomic mass (much later corrected to atomic number), were found to exhibit periodic behaviour. This was one of early chemistry’s major achievements. In the periodic table, these systematic variations in the properties of the elements change slowly down a column and also along a row. We are no longer dealing with 90-plus individuals but with a system that allows extrapolation of the properties of one element from those of others.
Missing elements were defined according to their predicted properties, which were later confirmed when they were found. Additional elements at the end of the table had their properties extrapolated from within the table before their artificial production. The Periodic Table was a change as dramatic as switching from doing arithmetic with Roman numerals to doing it with Arabic numbers. Only much later was a rational basis for the periodic table provided by an understanding of the structure of the atom in terms of protons, neutrons and electrons. Now is a good time to listen to Tom Lehrer’s Song of the Elements.1
Fig. 1.1. The periodic table of elements.
Source: https://en.wikipedia.org/wiki/Theodor_Benfey.
A chemical name must be unambiguous
The naming of chemicals can be confusing. Where purist chemists say ‘ethyne’, welders say ‘acetylene’. Linnaeus introduced the two-part nomenclature into Botany, such as Bellis perennis to describe a common daisy, and this approach was in turn adopted into systematic inorganic chemistry, thus magnesium sulfate for Epsom salts. In this book, we’ll stick to mainly talking about daisies but include a section on chemical nomenclature to aid those interested (see Appendix 1). Treat chemistry and its associated nomenclature like another language and recognise that it will take time to develop and master.
In order to have an unambiguous precise name for a chemical substance, a system has been devised to provide a systematic name that will allow us to describe any substance. These names are built up according to strict rules. Each compound has only one correct systematic name, but there is more than one system! The name conveys the complete information about the detailed structure of the compound: it is a stylised written description of the chemical structure. For example, ‘Aspro’, is a brand name; ‘aspirin’ or ‘acetylsalicylic acid’ is the generic term, and ‘2-(acetyloxy)benzoic acid’ is the IUPAC systematic name. To ensure that the chemical can be tracked down when required, each one is also given a sort of ‘tax file or social security number’ called a Chemical Abstract Service number CAS. The CAS number that number corresponds to 2-(acetyloxy)benzoic acid is 50–78–2. This is particularly important because chemicals appear under a variety of aliases – some more logical than others – but the CAS number links them all. The correct naming of chemicals is very complex and difficult even for professional chemists.
Chemical formulae and diagrams
Chemical formulae and diagrams are symbolic representations of the composition and structure of molecules and compounds. There are a variety of conventions for them, depending on the sort of information to be conveyed.
Level 1 information – empirical formula
At the lowest level of information is the empirical formula, which lists only the ratio of the different atoms present in the molecule and tells us nothing about the structure. For example, for acetic (ethanoic) acid, commonly known as vinegar when diluted with water, has the empirical formula CH2O.
Level 2 information – molecular formula
The molecular formula for acetic acid lists the actual number of different atoms in one molecule, rather than the empirical ratio. For acetic acid this is C2H4O2.
Level 3 information – group formula
The group formula places atoms together in groups that correspond to the grouping in the actual molecule and gives some indication (using prefix symbols) of how the groups fit together. For example, the group formula for acetic acid is CH3COOH. As you become more familiar with them, these group formulae can give a fairly complete (marginally ambiguous) description of the structure.
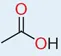
Level 4 information – condensed structural diagram
The condensed structural diagram, the most common form of line diagram, gives a two-dimensional representation of a three-dimensional structure. It leaves out a lot of the atoms, but their presence is implied by the shorthand conventions. For acetic acid, the condensed structural diagram is as shown in Fig. 1.2.
Fig. 1.2. Condensed structural diagram for acetic acid.
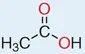
Level 5 information
The full structural diagram for acetic acid is shown in Fig. 1.3.