![]()
1
The fantastic nature of matter
In a book whose main aim, as outlined in the Preface, is to describe the fundamental nature of life, you could reasonably ask why it should start with an account of the atomic nature of matter, meaning anything in the universe that occupies space.
Everything is made of atoms, including all life forms. The existence of the phenomenon we know as life is due to the nature and properties of atoms, so this is a good place to start in understanding what life is. A bonus is that the story of how atoms came to exist on Earth is one of the most exciting in science.
The name ‘atom’ derives from the Greek word atomos, meaning indivisible. The Greek philosopher Democritus arrived at the concept that everything – all solids, liquids and gases – is made of minute particles that cannot be divided into smaller units. In other words, if you could cut up any matter into smaller and smaller pieces you would ultimately arrive at particles that you could not subdivide any further. Atoms were envisaged more or less as hard solid little balls. Although the concept of atoms has been around for so long, it is only in the last two centuries that they have been scientifically studied, and only in the last two decades that anyone has actually seen them as images using new microscopic techniques. They are indescribably small – the number in a few drops of water is approximately 10 followed by 20 zeros (1021).
Of the 92 naturally different atoms in the universe, only around 25 are found in your body, some in very small amounts. The four elements hydrogen, carbon, nitrogen and oxygen make up about 99% of the total number. The term ‘element’ is used to refer to a substance that is made from one type of atom and cannot be broken down by chemical means. The atoms in living organisms are the same as the corresponding ones in non-living matter. A carbon atom in your body is the same as a carbon atom in chimney soot or anywhere else in the universe, and the same applies to all atoms. So what then is the nature of life? What makes the difference between non-living and living matter?
We can talk about life as a single process. This may seem surprising in view of the vast variations found in life forms. The French Nobel prizewinner Jacques Monod famously expressed it as ‘what holds for Escherichia coli is true for an elephant’. He meant that the basic chemistry of the microscopic bacterial cell E. coli, which lives in the human gut in countless numbers, is much the same as the chemistry of cells in an elephant. The similarities far outweigh the differences.
Life is basically a chemical process. And this is true for all living organisms. The reason for this is that there was a single origin of life from which all life has developed over approximately 3 billion years.
The salient feature of life is that it reproduces itself. The first form of life must have been a relatively simple self-reproducing collection of atoms on the primeval Earth, and since then life has been handed down from generation to generation. To do this a system of passing on information had to be devised which determines that the offspring resembles the parents. In other words, there had to be a genetic system.
The basic mechanism of the genetic system is the same in an E. coli cell as in a human, a whale, a tree, an insect, or any life form you care to nominate. There are superficial differences in detail between the process in E. coli and higher forms, but these do not affect the essentials. It seems that life became locked into the method of passing on genetic information to offspring at a very early stage of evolution.
The process of evolution has also superimposed variations on this basis of life that enable organisms to exploit environmental niches. Plants, for example, developed the ability to use sunlight as an energy source; birds adapted to the air and whales to the sea, but it has not changed the basis of life common to all.
There are also fundamental laws of nature that life had to conform to; once again the remarkable solutions to these problems are much the same in all life. We will come to discuss these in subsequent chapters.
The living unit of organisms is the cell
Living cells are, with the inevitable rare exceptions, microscopic structures surrounded by a membrane (Fig. 2.2). In your body there are many trillions of cells. The E. coli cell is about 1000 times smaller in volume than animal and plant cells.
Why are cells so small? They have to communicate chemically with the outside world through the membrane. There are mechanisms for transporting chemicals in and out of the cell and for conveying signals into it. For example most chemical messengers such as insulin do not enter cells but deliver a signal to it via receptors in the membrane, which act like aerials. What has this got to do with cell size? There has to be an adequate area of membrane to service the needs of the cell. Minute cells satisfy this requirement because they have a lot of surface in proportion to their volume, but if you increase cell size the volume increases much more rapidly than does its surface area. You quickly arrive at the point at which the membrane area is inadequate to support the needs of the cell. So living cells have to be tiny.
A bacterium like E. coli is free living, but to build larger organisms cells are aggregated into more complex organisms. This requires a regulatory system to coordinate the activities of individual cells to the needs of the organism as a whole. In an animal such as a human this becomes very complex indeed. The number of human cells that have to be kept in step with one another vastly outnumbers the whole of the population on Earth, so you can see this is no small problem. Cancer is the result of a cell no longer observing the regulatory rules and going its own way.
So, a living cell is a chemical device. Obviously many will say that life is more than chemistry, but I am talking about the process of life and, whatever views are held, there has to be a mechanism. To most people without any training in chemistry this statement will not be meaningful, but with a simple knowledge of atoms it is possible to understand the nature of life at a profound level.
Biology is dependent on chemistry
Chemistry is essentially a description of how atoms react with one another, and from this life can be seen as the outcome of a large number of chemical reactions occurring in an organised manner in living cells. Here is the basis for the connection between biology and chemistry.
In the case of humans there are somewhere of the order of tens of thousands of different chemical reactions. This involves rough estimates but the number is very large. Behind these chemical reactions there are several fundamental problems that life had to solve to conform to the natural laws of the universe. What these problems are and the way in which they were solved is mainly what this book is about.
The thousands of individual reactions in life are details which do not have to be described for you to understand the principles of the living process. Leaving these aside, we are left with the big picture of life – the major concepts that determine the nature of life and its relationship to the laws of the universe.
The first step is to look at the nature and properties of atoms. A good point to start is to discuss where atoms and the universe came from in the first place.
The Big Bang
Astrophysicists have discovered that 13.7 billion years ago there were no atoms and no universe. At that date the universe came into existence with an indescribable explosion. The physicist Fred Hoyle, in Cambridge, argued tenaciously for an alternative theory that the universe has always existed and always will exist, known as the steady-state universe. He dismissed the explosion theory, describing it, rather derisively, as the ‘Big Bang’, but evidence rapidly accumulated that the explosion theory is correct. The term Big Bang summarised it so well that it was adopted by its supporters and is now used by everyone.
To return to the Big Bang, what was it that exploded? No one knows, but it was from a source of infinite density and energy content. It contained all the components and energy of the present entire universe. If you reflect that there are hundreds of billions of galaxies in the universe, each with hundreds of billions of stars, it was some condensation! What was there before this ‘thing’? Physicists tell us that the question is invalid because there was no ‘before’, since Einstein’s relativity theory shows that time was created at the Big Bang. So was space. The explosion of the Big Bang is not to be thought of as ejecting things into space but as the creation and expansion of space. The space between objects expanded, so that in the course of the explosion they became increasingly separated as the universe expanded.
The Big Bang explosion is believed to have produced a soup of concentrated energy and fundamental particles, the ultimate components of atoms: the temperature initially in the first few seconds was too high for any atomic structures to exist. As the explosion rapidly expanded, it cooled to a point at which subatomic particles were formed and these assembled further into atoms of hydrogen with smaller amounts of helium. Hydrogen is still the predominant atom of the universe.
What are atoms made of?
Although in classical terms atoms cannot be further subdivided, physicists using atom-smashing machines have released smaller subatomic components from them. There are three types of these components, called protons, neutrons and electrons. All atoms are made of them. Hydrogen has one proton and one electron; uranium, the heaviest atom found in nature, has 92 protons and 92 electrons. As you go down the list of atoms from hydrogen to uranium each element has one more proton than the one above it in the list, with the number of electrons always being the same as that of protons. Thus helium, the second element in the list, has two of each. The next one, lithium, has three of each, and so on. Elements that you will be familiar with are carbon, with six protons and electrons, phosphorus, with 15 of each, sulfur with 16, and iron with 26. The number of protons in an element, known as the atomic number, defines what the element is. An atom with six protons is carbon, and so on. We shall talk about neutrons later.
A proton has one positive electrical charge and an electron has one negative charge of the same size, so atoms are electrically neutral. All my professional life I have been familiar with electrical charges in biochemistry, where they are very important, but when I sat down to explain what they are I realised that I didn’t know. Most of us take them for granted. A physicist revealed to me that nobody knows what they are. They are unexplained fundamental properties of nature. It is the way nature is. We identify charges by the way they behave in an electric field. Positive charges repel one another but are attracted to the negative pole, and negative charges repel one another and are attracted to the positive, but which is positive and which is negative is an adopted convention. So, in summary, we know how they behave and their importance in chemistry, but not what they are.
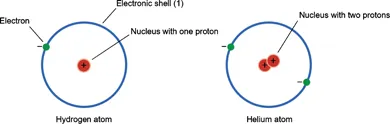
Neutrons, as their name suggests, have no electrical charge, but they have almost the same mass as protons. (Mass on Earth equals weight, but weight depends on the gravitational pull while mass does not. Your weight on the Moon would be much less than on Earth but your mass would be the same because it depends only on the amount of matter making up your body.) All atoms except normal hydrogen have neutrons, but the numbers vary. They occur in the tiny atomic nucleus tightly combined with the protons, and the mass of an atom is almost entirely due to these two components. Electrons, which have only one two-thousandth the mass of a proton, are outside the nucleus. Figure 1.1 shows diagrams of a hydrogen atom and a helium atom.
The hydrogen atom, with one proton, has one unit of mass; all the rest of the elements have masses that are multiples of this. Since the number of neutrons in an element can vary, atoms of a given element may have a variety of atomic masses. These are known as isotopes. Deuterium is the name given to hydrogen with a single neutron. It is also known as heavy hydrogen, giving rise to heavy water. Neutrons allow the positively charged protons to pack together in the nucleus, with larger nuclei needing more neutrons to hold them together. When a nucleus is very large the neutrons struggle to overcome the repulsion between the protons; this explains why a particular isotope of uranium (235) can be used in nuclear fission bombs in which the atom splits into two smaller ones with the release of tremendous amounts of energy.
Figure 1.1: Diagram of a hydrogen atom and a helium atom. Note that the circles represent the pathway the electrons take as they move around the nucleus (the electron shells). The shells are not physical structures; atoms have no visible boundaries but have an electronic barrier which prevents them invading each other’s space (see text).
Where did atoms other than hydrogen come from?
Atoms are made in the stars. On Earth they are eternally unchanged and unchangeable, with the minor exceptions of the decay of radioactive atoms into other elements and a few alterations made by atomic physicists. Production of new elements from hydrogen is made by fusions between nuclei of atoms, and this can only occur at the high temperatures and pressures inside stars. Stars form from clouds of hydrogen which increase in size as gravitational attraction captures more hydrogen atoms. The tremendous gravitational pressure at the centre of the mass heats up the interior until it reaches the critical point at which protons of hydrogen atoms are able to fuse together, despite electrical repulsions, to produce helium, and in the process liberate tremendous amounts of energy. In other words, at this point the star lights up.
The process does not stop at production of helium. In stars like our Sun, further nuclear fusions between atoms produce elements up to atomic number 26, which is iron, but the process stops there. Further conversion of iron to elements with higher atomic numbers requires much higher temperatures than occur in the Sun. There is only one known type of event in the universe where this occurs: in the explosion as a supernova of a star ten times as massive as the Sun. In stars, the heat production by fusion of hydrogen and other elements counteracts the enormous internal gravitational pressures, but as the star runs out of fuel gravity gets the upper hand and the star contracts. In a small star like our Sun, repulsion between the atoms will eventually counter the effect of gravity, but a more massive star can contract further, developing indescribably high temperatures and pressures at the centre that result in the external layers being blasted into space in a gigantic explosion. For a few weeks the exploding star may radiate as much energy as the entire galaxy or as much as the Sun will radiate in its entire life. During this process, the elements heavier than iron, up to uranium the heaviest, are produced.
The series of nuclear reactions which produce all the elements have been worked out in detail. The debris from the explosion was scattered into the clouds of hydrogen gas. Ultimately new stars are produced by gravitational attraction; one such is our Sun, accompanied by its planets, one of which of course is the Earth. This improbable-sounding series of events was the origin of all the 92 elements on Earth. Fred Hoyle, inventor of the term ‘Big Bang’, played a prominent part in this epic piece of research. He was a brilliant physicist who also became well known for his science fiction novel The Black Cloud.
The structure of atoms
When scientists realised that atoms contained positively and negatively charged components, they also realised that atoms had a structure that had to be explained. Initially there was the so-called plum pudding atomic model in which sub-atomic components were dotted around the atom in some undefined medium rather like fruit in a plum pudding.
A major advance in understanding the structure of the atom was made by Ernest Rutherford, a New Zealander. After graduating in mathematics and physics he was awarded an 1851 Exhibition Scholarship. These scholarships were inspired by Prince Albert and funded by the profits of the Crystal Palace indu...