eBook - ePub
Biomedical Physics in Radiotherapy for Cancer
Loredana Marcu,Eva Bezak,Barry Allen
This is a test
Share book
- 448 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biomedical Physics in Radiotherapy for Cancer
Loredana Marcu,Eva Bezak,Barry Allen
Book details
Book preview
Table of contents
Citations
About This Book
The scientific and clinical foundations of Radiation Therapy are cross-disciplinary. This book endeavours to bring together the physics, the radiobiology, the main clinical aspects as well as available clinical evidence behind Radiation Therapy, presenting mutual relationships between these disciplines and their role in the advancements of radiation oncology.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Biomedical Physics in Radiotherapy for Cancer an online PDF/ePUB?
Yes, you can access Biomedical Physics in Radiotherapy for Cancer by Loredana Marcu,Eva Bezak,Barry Allen in PDF and/or ePUB format, as well as other popular books in Medicine & General Health. We have over one million books available in our catalogue for you to explore.
Information
Topic
MedicineSubtopic
General Health1 Interactions of radiation with matter
1.1 IONISING RADIATION
Radiation (i.e. energy that is radiated and propagated in the form of rays or waves or particles) is classified into two main groups: non-ionising and ionising, depending on its ability to ionise matter. Ionising radiation is radiation with enough energy to be able to remove tightly bound electrons from the orbit of an atom, causing the atom to become charged or ionised. Some examples of ionising and non-ionising radiation are listed in Table 1.1.
Atoms and molecules are electrically neutral. This means the number of negatively charged electrons is exactly equal to the number of positively charged protons. Most of the matter around us is electrically neutral. However, when there is an ionising radiation source available, atoms or molecules can gain or lose electrons and acquire a net electrical charge. This process is called ionisation. The minimum energy required to ionise an atom, ranges from a few electron Volts (eV) for alkali elements to 24.5 eV for helium (noble gas). Specific forms of ionising radiation include: a) particulate radiation, consisting of atomic or subatomic particles (electrons, protons, etc.) which carry energy in the form of kinetic energy or mass in motion and b) electromagnetic radiation, in which energy is carried by oscillating electrical and magnetic fields propagating at the speed of light. The medium traversed by radiation can be ionised directly or indirectly.
Table 1.1. Examples of ionising and non-ionising radiation.

Directly ionising radiation deposits energy in the medium through direct Coulomb interactions between the directly ionising charged particle (e.g. an alpha particle) and orbital electrons of atoms in the medium. Indirectly ionising radiation (photons or neutrons) deposits energy in the medium through a two step process: I) a charged particle is released in the medium (photons liberate atomic electrons or generate positrons, neutrons release protons or heavier ions), II) the released charged particles deposit energy to the medium through direct Coulomb interactions with orbital electrons of the atoms in the medium.
Photon radiation is further classified as below:
- Characteristic X-rays: result from electron transitions between atomic shells
- Bremsstrahlung X-rays: result from electronnucleus Coulomb interactions
- Gamma rays: result from nuclear transitions
- Annihilation quanta: result from positron-electron annihilation
Both directly and indirectly ionising radiations are used in treatment of cancer. In some cases non-cancerous tumours are also treated. The branch of medicine that uses radiation in the treatment of disease is called radiotherapy or radiation oncology.
1.2 X-RAYS
X-rays were discovered by Roentgen in 1895 in Germany during his study of ultraviolet light in the discharge tube (stream of electrons). BaPt cyanide filter paper placed in the vicinity of the discharge tube started glowing due to fluorescence. Roentgen concluded that another type of radiation must have been produced, presumably during the interaction of electrons with the tube’s glass walls. This radiation could be detected outside the tube and also caused exposure of photographic plates and ionised a gas. He named this new radiation X-rays. They were subsequently extensively investigated and eventually classified as one form of electromagnetic radiation. Within months they began to be used for medical purposes.
The main properties of X-rays are listed below. X-rays:
- Are electromagnetic waves of short wavelengths, e.g. X-ray energy of 1 MeV corresponds to wavelength, λ, of about 1.24 pm. Short wavelengths are used for studies of diffraction of X-rays from crystalline structures, known as X-ray crystallography.
- Travel at or near the velocity of light.
- Travel in straight lines and are unaffected by electric and magnetic fields (as they have zero electric charge).
- Various normally opaque materials are transparent to X-rays. The degree of transparency depends on their atomic number, Z, and photon energy. High Z materials are more absorbing than low Z and any material is more transparent to higher energy photons.
- Obey inverse square law (i.e. their intensity reduces as the square of the distance from the source).
- Are generated by high voltages in X-ray tubes in which an electron beam in vacuum is stopped by a metal anode.
- Do not readily reflect or refract.
- Discharge electrified bodies and ionise gases (make them conductive).
- Have energy and momentum and undergo interactions with electrons and nuclei of the medium (absorption, scatter), e.g. photoelectric effect, Compton scatter (incoherent), Thomson scatter (coherent) and pair production.
- Main sources of X-rays are bremsstrahlung radiation of continuous energy distribution and characteristic atomic radiation of discrete energy.
- May follow α and β decay.
- Can be polarised by scattering, e.g. two carbon blocks behave as polariser and analyser (i.e. they show properties of electromagnetic waves).
- Reflect very little except at low energies and off smooth metal surfaces at grazing incidence.
- In diagnostic radiology (imaging) peak voltages of ~50–150 kV are used to produce X-rays, while in radiation therapy corresponding voltages are in the range of 4–25 MV.
X-ray photons typically have the wavelengths of the same size or less than the diameter of a single atom, about 0.1 nm (or 0.1 × 10–9 m). X-rays can penetrate through soft material such as flesh. Much of the X-ray spectrum overlaps with gamma rays. X-rays and gamma rays mainly differ only in the manner of their production. X-rays are produced by electrons whilst gamma rays are produced by nuclei (see Figure 1.1).
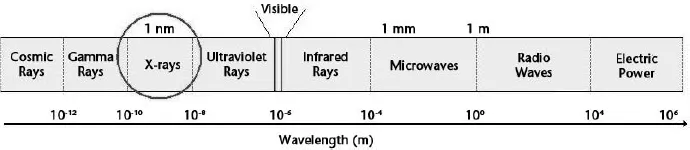
Figure 1.1. Wavelengths of various types of electromagnetic radiation.
1.2.1 Characteristic X-rays
Characteristic X-rays are the characteristic atomic line spectra. An electron with kinetic energy, Ee, passing through a medium will interact with atoms of the material. In most cases it will cause ionisation, i.e. the ejection of the outer shell electrons. Occasionally, ...