
eBook - ePub
Petroleum Spills in the Marine Environment
The Chemistry and Formation of Water-In-Oil Emulsions and Tar Balls
This is a test
- 158 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Petroleum Spills in the Marine Environment
The Chemistry and Formation of Water-In-Oil Emulsions and Tar Balls
Book details
Book preview
Table of contents
Citations
About This Book
This book covers research completed between 1981 and May 1985 and includes: reviews of recent studies, sitings and investigations at spills-of-opportunity as well as results of recent arctic and sub-Arctic oil weathering experiments and observations on the behavior of crude oil in the presence of ice. Topics covered include the following: laboratory studies of formation and stability of water-in-oil emulsions; selected case histories of the more detailed chemistry studies of mousse behavior and long term fate in near-coastal and open ocean oil spills/blowouts; tar ball formation and distribution; and algorithms and computer programs to simulate the formation of water-in-oil emulsion.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Petroleum Spills in the Marine Environment by James R. Payne in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Civil Engineering. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
INTRODUCTION
A better understanding of the phenomenon of water-in-oil emulsification and tar ball formation from petroleum spills at sea is critical to our ability to predict, control, and mitigate the environmental impacts of petroleum hydrocarbon spills in marine and coastal waters. Stable water-in-oil emulsions or “mousse” complicate clean-up strategies and logistics because the more viscous emulsions present formidable problems in skimming, pumping, and recovery operations. Emulsified oils also require an inordinate amount of space in transport and intermediate storage due to the increase in volume from water incorporation. Additionally, in the final stages of disposal, certain water-in-oil emulsions may resist more convenient and conventional disposal mechanisms such as burning.
When crude oil and many refined products are released at sea they are subjected immediately to a series of weathering processes including: spreading; evaporation and dissolution of selected lower molecular weight components; dispersion of whole oil droplets into the water column; coalescence and return to the surface slick of those droplets with entrapment of seawater; photo-, microbial- and auto-oxidation; and emulsification and tarball formation. The rates of these concomitant processes are inextricably linked (and in some cases compete) with one another. They are also dependent on the type and amount of oil spilled (component concentrations), environmental conditions (water and air temperature, wind speed, and turbulence regime -- sea state), and man’s own efforts to control or disperse the slick through the application of dispersants/demulsifiers and/or sinking agents.
Most crude oils and refined petroleum products have specific gravities less than one and will not readily sink after initial release. However, the combined effects of natural and enhanced weathering processes alter the density, viscosity, pour point, and volume of these products such that ultimate cleanup and containment strategies must be capable of handling an incredible variety of situations. During formation of water-in-oil emulsions, products of higher density and viscosity which contain up to 70 to 80% water (dispersed as sub-micrometer to 50 micrometer droplets in the continuous oil phase) can be generated and, as such, burning may become more difficult due to the high water content and chemical dispersion may prove to be impossible. Because of their greater density, however, water-in-oil emulsions, and tar balls generated from such mousse, may be more susceptible to submersion which ultimately enhances dispersion of oil slicks.
Numerous investigators have suggested that mousse formation and stability are influenced by the presence of surfactant materials such as asphaltenes, waxes, organometallics, and nitrogen, sulfur and oxygen (NSO) compounds which are important in preventing water-water droplet coalescence within the emulsion. It has been found that the more viscous oils tend to form more stable emulsions and water-in-oil emulsions form more rapidly under lower temperature (higher viscosity) than higher temperature conditions. Turbulence has also been demonstrated to be critical in mousse formation. At this time, however, no single explanation accounts completely for all of the observations, and not all of the mechanisms of mousse formation and stability are understood.
In this review, attempts have been made to provide a broad view of the subject. Topics include discussions of laboratory and field test-tank (wave and mixing chamber) experiments used to examine specific factors associated with mousse formation. The importance of oil composition and different turbulence regimes, as well as discussions of studies of mousse formation and behavior in real spill situations are included. Several major spill incidents are considered with regard to observed and documented mousse and tarball formation, stability, and fate. Whenever possible, correlations are made between real spill situations and laboratory simulations. The occurrence, distribution, and chemistry of tarballs from other sources are briefly considered; however, not as much emphasis has been placed on this subject due to the highly variable levels of tarballs in the world’s oceans and their somewhat limited long-term environmental impact.
Finally, a brief review presents recent attempts to simulate mousse formation and behavior through mathematical and computer modeling. These models generally are coupled to, or based upon, laboratory wave tank and mixing chamber experiments, although several attempts to model field observations with computer predictions (hind casting) have been completed.
CHAPTER 2
LABORATORY STUDIES OF FORMATION AND STABILITY OF WATER-IN-OIL EMULSIONS
BACKGROUND
Before undertaking a discussion of water-in-oil emulsion formation and stability, it is necessary first to review several general aspects of emulsions and emulsification. A more comprehensive treatment of the subject is presented by Twardus (1980). In general, an emulsion is defined as two immiscible liquids wherein droplets of one phase (the dispersed or internal phase) are encapsulated within sheets of another phase (the continuous or external phase). When crude oil or petroleum products are spilled at sea, two basic forms of emulsions are possible. The first is an oil-in-water (O/W) emulsion in which oil droplets are dispersed and encapsulated within the water column. The second is a water-in-oil (W/O) emulsion in which droplets of water are dispersed and encapsulated within the oil. This second mixture is generally referred to as mousse in the literature. For either type of stable emulsion to form between two liquids, three basic conditions must be met: (1) the two liquids must
be immiscible or mutally insoluble in each other; (2) sufficient agitation must be applied to disperse one liquid into the other; and (3) an emulsifying agent or combination of emulsifiers must be present.
During emulsification, the interfacial area between two liquids increases. Liquids tend to minimize this surface area, therefore, an emulsifying agent and work (or energy) are required for emulsification to proceed. In theory, the amount of energy required to increase the surface area can be calculated if the interfacial tension between the two liquids is known (Becher, 1955). In open ocean and coastal oil spills, sufficient energy to satisfy this requirement typically is provided by wind, waves, and currents. Nevertheless, stable water-in-oil emulsions also have been observed to form with certain oils even on very calm seas. The emulsifying agent may be any surface active substance which can form a thin interfacial film between the two liquids and maintain the emulsion by minimizing the contact, coalescence, and aggregation of the internal dispersed phase. For emulsions to form in the absence of external agitation, the interfacial tension between the two liquids should be reduced to approximately 0.5 dynes/cm, whereas only approximately 5 dynes/cm are needed for emulsions formed with agitation. The surfactant should surround the dispersed droplet as a nonadhering film and should have a molecular structure in which the polar end is attracted to the water and the non-polar end is attracted to the oil. Surfactants should be relatively more soluble in the external phase so that they are readily available for adsorption around the internal phase. These surfactants also may impart an electro-kinetic potential and influence the viscosity of the emulsion formed. Finally, the surfactant material must stabilize the emulsification process while present in relatively small quantities.
Depending on the chemical composition of the surfactant, emulsion stability can either increase or decrease. For example, materials containing mono-valent ions have been shown to stabilize oil-in-water emulsions, whereas surfactants containing poly-valent ions can stabilize water-in-oil emulsions. A number of materials are present in crude oils which stabilize water-in-oil mixtures (these will be discussed later in greater detail). In general, however, unrefined oils have relatively higher portions of water-in-oil emulsifying agents than oil-in-water emulsifying agents. Thus, while both types of emulsions can form in petroleum spills, the majority of the emulsion would be the water-in-oil type. Furthermore, oil-in-water emulsions are inherently unstable, and they have been shown to revert to water-in-oil mixtures. Effects of shear rate, temperature, and oil concentration on the formation of oil-in-water emulsions were studied by Mao and Marsden (1977) using California crude. They noted that increases in temperature and/or oil concentration enhanced the conversion of oil-in-water emulsions to water-in-oil emulsions.
In water-in-oil emulsions, asphaltene substances, porphyrin complexes, and waxes act as natural emulsifying agents stabilizing W/0 mixtures (Berridge et al., 1968a, 1968b; Cairns et al., 1974; Canevari, 1969; Frankenfeld, 1973). Presumably these agents provide the required film around the water droplets which resists rupture, thus preventing water-water coalescence (Canevari, 1982). The size distribution of water droplets in W/0 emulsions, discussed in greater detail later, is also important.
The stability of water-in-oil emulsions is dependent on a variety of factors, including: the presence or absence of the emulsifying agent, viscosity (influenced greatly by temperature), specific gravity, water content, and the age of the emulsion. Essentially, the stability of a W/0 emulsion could be defined as the resistance by the dispersed water droplets against coalescence. This definition is based upon the phenomenon of Brownian movement, such that the emulsions having a high specific gravity and viscosity would tend to be more stable because movement of the water droplets theoretically would be reduced. As noted above, increases in temperature which result in reductions of viscosity or increases in the water droplet concentration in the continuous petroleum phase would increase the probability of collision and coalescence, thus destabilizing water-in-oil emulsions.
SPECIFIC STUDIES
A number of laboratory experiments have been undertaken in mixing chambers and wave tanks to study the formation and behavior of mousse. Evaporation and dissolution typically were allowed to occur to simulate ambient environmental conditions. In almost all instances, hydrocarbons with molecular weights less than nC-11 to nC-12 (distillation range 200° to 225°C) were lost during the initial stages of weathering, as observed in studies of open ocean and nearcoastal spills. The results of these studies and the physical properties and selected chemical characteristics of the crude oils and resultant water-in-oil emulsions are summarized in Table 1. Table 2 lists several additional oils which have demonstrated water-in-oil emulsification tendencies, but for which only limited data are available.
Table 1 Mousse formation experiments using a variety of fresh and artificially weathered (topped) crude oils in laboratory, outdoor test tank, and field experimental spills.
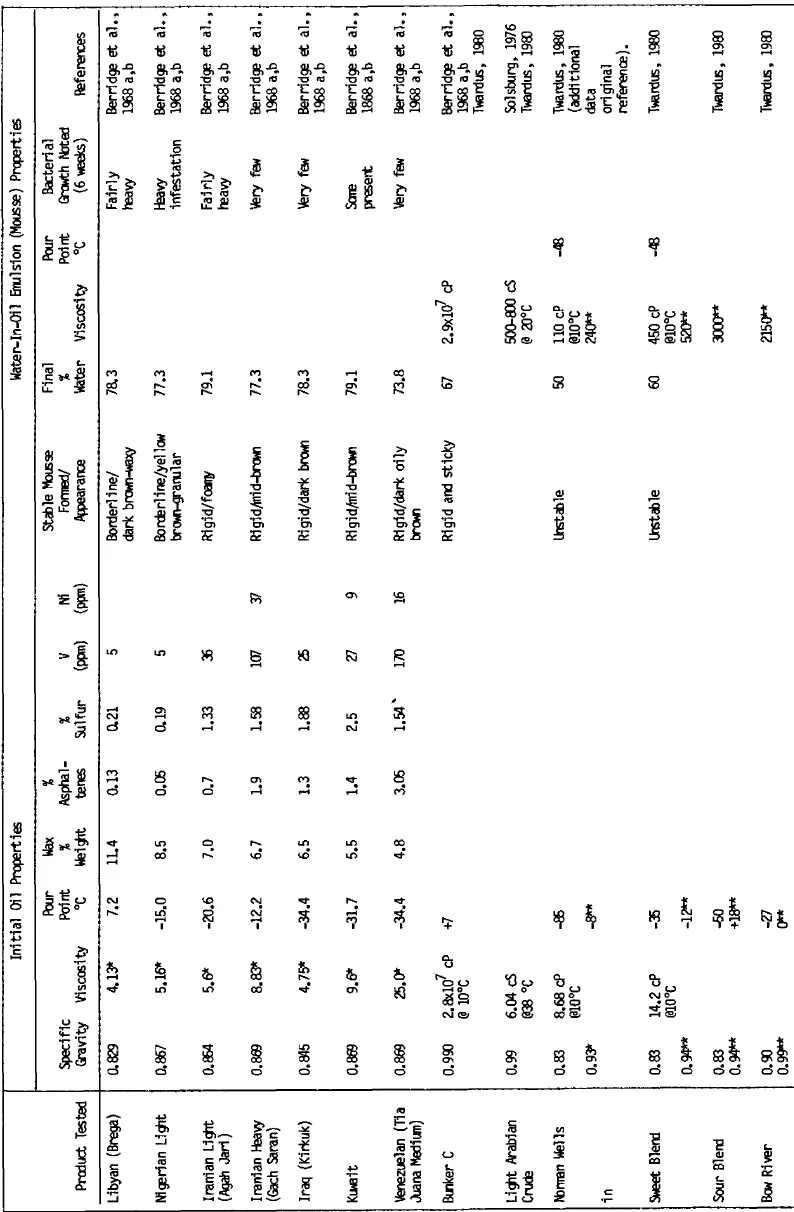
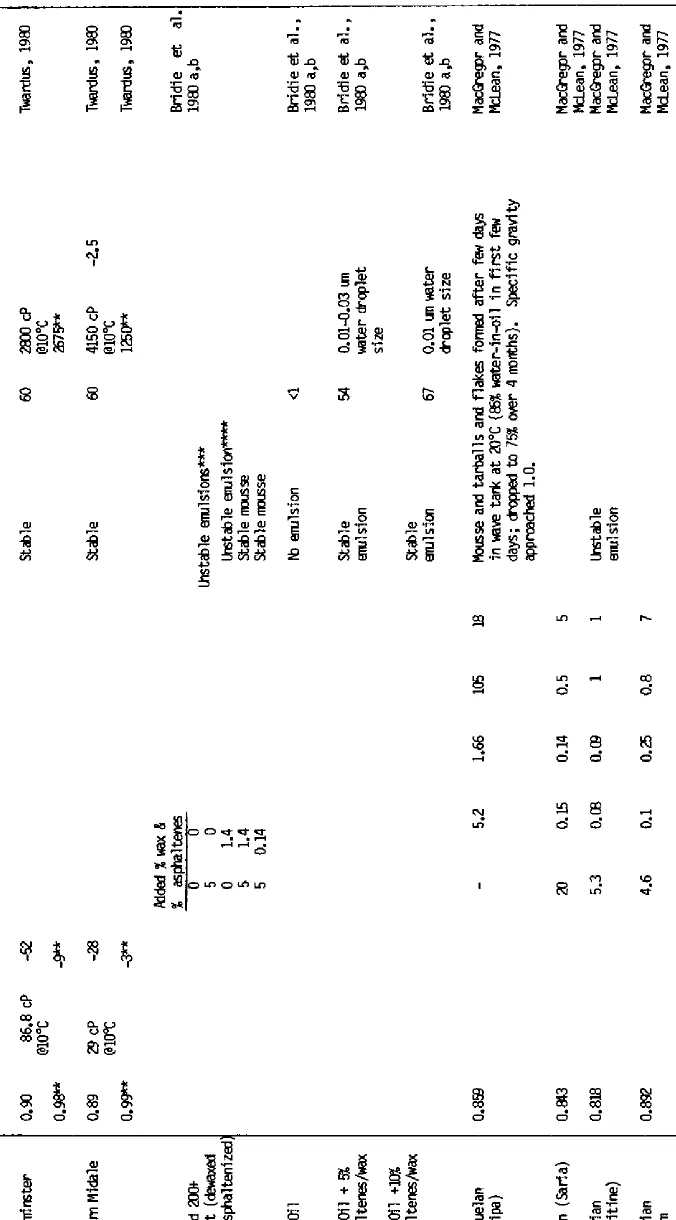
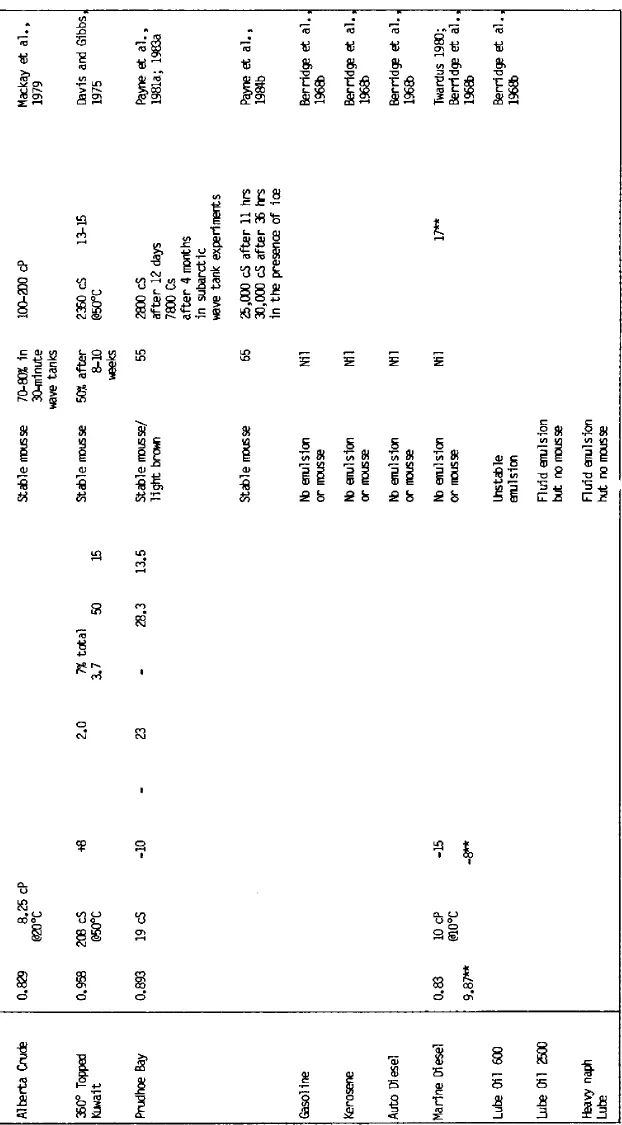
* Kinematic viscosity (cS) at 100°F
** Specific gravity and pour point after 4 weeks pan evaporation under atmospheric conditions (no water added except for occasional precipitation).
*** 93% of water shed after standing 15 minutes
**** 88% of water shed after standing 15 minutes
TABLE 2
Other Oils Which Have Demonstrated Water-in-Oil Emulsion Tendency (from Bridie et al., 1980a)
Other Oils Which Have Demonstrated Water-in-Oil Emulsion Tendency (from Bridie et al., 1980a)
Crude Oil | Source | Mousse Formed | Flow Properties | Spreading on water at 10° C |
Brent | N Sea GB | + | Viscous | – |
Ekofisk | N Sea Norway | + | Unstable | – |
Auk | N Sea GB | + | Viscous | – |
Kuwait | Kuwait | + | Paste | – |
Nigerian Medium | Nigeria | + | Low Vise | + |
Qatar Marine | Qatar | + | Viscous | – |
Cabimas | Venzuela | – | – | + |
Iranian heavy | Iran | + | Paste | – |
Berridge el al. (1968a, b) studied the effects of chemical composition of the starting crude and evaluated mousse formation potential and stability for seven crudes that were selected to give a representative sampling of oils likely to cause marine pollution. Specific gravities of the crudes ranged from 0.829 for Libyan (Brega) crude to 0.896 for the Venezuelan (Tia Juana medium) crude. Sulfur contents for the crudes range from 0.2 to 2.5%, and kinematic viscosities at 100°F ranged from 4.13 to 25 centistokes. Pour points for the selected oils ranged from –34° to +7°C, and wax contents were found to vary inversely with the specific gravity, ranging from a high of 11% by weight for the Libyan crude to 4.8% by weight for the Tia Juana crude. Additional characterization data are presented in Table 1. Asphaltenes were found to increase in weight percent from 0.13 to 3.5 and were roughly inversely proportional to the wax content. Vanadium content increased with the increase in specific gravity and percent asphaltenes. Residues with components having boiling points greater than 370°C ranged on a weight percent basis from 35 to 57% for the crudes studied, and interestingly, the residue pour points decreased from 100° to 50°C from the light to the heavier crudes. Thus, when spilled at sea, crudes such as the Libyan Zelten (Brega) and the Nigerian light, which have fairly low percentage ranges of residues greater than 700°, will be removed relatively rapidly by evaporation. Evaporation is particularly effective for weathering Zelten crude which contains 31% by weight of components which boil below 200°C. Heavier crudes, such as the Tia Juana medium (78% residue boiling greater than 370°C), contain only a small fraction which distills at low temperature; thus, they would evaporate very slowly and would not be expected to weather appreciably by evaporative processes.
The weathered residues obtained from the evaporative processes acting on all the oils studied had higher specific gravities, viscosities, sulfur, metal, and wax content than the original crudes. For example, Kuwait crude contains approximately 27% by weight residual materials with a boiling point above 1,000°C and a specific gravity of 1.028. Similarly, an Iranian heavy crude residue has a specific gravity of 1.027. Therefore, both of these residues have densities greater than seawater (1.025 g/cm3) and would be expected to sink relatively easily in the marine environment.
Rigid, stable emulsions were formed with most of the oils tested, with the exception of Brega and Nigerian light crude which were classfied as marginal. Emulsion colors ranged from mid-brown to dark oily-brown to a yellow brown granular substance with the Nigerian light crude. Residual fuel oil (Bunker C) also formed a stable mousse. In contrast, no mousse could be generated using distillates such as gasoline, kerosene, and diesel oils. Lubricating fluids did not form stable “mousse”, but emulsions that were either unstable or fluid could be generated. In general, variation in the size of water droplets appeared to correlate with stability; the Brega and Nigerian crudes contained the largest water droplets and exhibited the least stability and greatest potential for the water and oil to separate into distinct phases. The more stable emulsions contained water droplets with diameters less than or equal to one micrometer.
The effect of salinity was also investigated by Berridge et al. (1968a, b). Mousse type emulsions were obtained with Kuwait crude and Tia Juana crude with water contents ranging from 74 to 80% regardless of salinity. A stable emulsion formed in all cases, but the appearance of the mousse ranged from a mid-brown for Kuwait crude with seawater to a midgray-brown using tap or distilled water. Tia Juana crude with seawater formed a very dark brown mousse, whereas the mousse formed with tap water and distilled water was nearly black. To evaluate other factors affecting mousse formation, oil and water were filtered through Whatman #1 filter paper to remove particulates above 100 microns, and in several instances the water was sterilized by the addition of 500 ppm dichlorophen. In general, these procedures did not affect the water content or mousse appearance.
Mousse stability was measured by placing 425 ml of mousse from the different crudes on glass plates and allowing them to weather naturally under environmental conditions. Identical samples were also placed in 2 gallon buckets and agitated from below with bubbles. Stable mousse on the glass produced an oil fringe while maintaining an overall rigidity, whereas less stable mousses deformed or slumped and flowed off the glass under the influence of wind, gravity, and rain. Estimated losses due to weathering of so-called beached mousse ranged from 10% for Kuwait crude to 80% for the Brega crude, which formed a much softer, waxy mousse. Nigerian crude oil mousse was very granular on the plate and formed a waxy or oily and foamy mousse. Microbial populations were observed to grow with Kuwait mousse, whereas very few bacteria were present for Tia Juana, Iraq, Kirkuk, and Gach Seran crudes. Heavy bacterial infestation was observed with the Nigerian ...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- 1. INTRODUCTION
- 2. LABORATORY STUDIES OF FORMATION AND STABILITY OF WATER-IN-OIL EMULSIONS
- 3. SELECTED CASE HISTORIES OF THE MORE DETAILED CHEMISTRY STUDIES OF MOUSSE BEHAVIOR AND LONG TERM FATE IN NEAR-COASTAL AND OPEN OCEAN OIL SPILLS/BLOWOUTS
- 4. TAR BALL FORMATION AND DISTRIBUTION
- 5. ALGORITHMS AND COMPUTER PROGRAMS TO SIMULATE THE FORMATION OF WATER-IN-OIL EMULSIONS
- 6. SUMMARY, CONCLUSIONS, AND CRITICAL CITATION REVIEW
- BIBLIOGRAPHY
- INDEX