
This is a test
- 366 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
Growth Regulation and Carcinogenesis discusses topics such as growth factors, including stimulators and inhibitors of proliferation; networks in proliferation regulation; differentiation-inducing factors; origins of neoplasia and their relationship to growth control; genetic alterations in cellular regulatory machinery; extrachromosomal phenomena; non-genotoxic carcinogens; immortalization and transformation of cells; and the role of cell production, cell function, and cell elimination in physiological growth control.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Growth Regulation and Carcinogenesis by Walter R. Paukovits in PDF and/or ePUB format, as well as other popular books in Biowissenschaften & Zellbiologie. We have over one million books available in our catalogue for you to explore.
Information
Section IV: Growth Regulation
Chapter 1
The Cyclin (Pcna) Probe of the Cell Cycle
Julio E. Cells and Peder Madsen
Table of Contents
I. Introduction
II. Cyclin (PCNA)
Acknowledgments
References
I. Introduction
The life cycle of eukaryotic cells is divided into four periods based on two landmarks, cell division at mitosis (M) and chromosomal DNA replication in S phase.1 At present, very little is known concerning the molecular mechanisms that control progression through the various phases.
Studies in this laboratory have for some years been dedicated to identifying cell cycle specific proteins that may be components of the pathways that regulate cell proliferation in human cells.2, 3, 4 and 5 In particular, we have focused our attention on identifying proliferation sensitive proteins whose rate of synthesis increases at or near the Gj/S transition border and that are common to all human cell types, as these are expected to be late obligatory components of the mitogenic response.2, 3, 4 and 5
In this short article, we shall review experiments leading to the identification of the DNA replication and cell cycle regulated nuclear protein cyclin,4,7, 8 and 9 also called proliferating cell nuclear antigen,10 or the auxiliary protein of DNA polymerase δ.11, 12 and 13
II. Cyclin (Pcna)
Cyclin (PCNA) is a proliferation sensitive and cell cycle regulated nuclear protein. Studies by Bravo and Celis7,14,15 using high resolution two-dimensional gel electrophoresis were among the first to identify proliferation sensitive proteins that may be cell cycle regulated. In particular, their studies identified a nuclear protein of apparent molecular weight of 36 kDa (see Figure 1) whose rate of synthesis correlated directly with the proliferative state of normal cells, and one that was synthesized at a higher rate by many transformed cells (see Figure 2).2,4,9 Cyclin (PCNA) was shown to be synthesized preferentially during the S phase of the cell cycle,7 increased synthesis starting late in Gl near the Gl/S transition border of the cell cycle. Analysis of the rate of synthesis of cyclin (PCNA) during two successive cell cycles of transformed human amnion cells (AMA) have shown that this protein is a true cell cycle regulated protein (Figure 3).16
Independent studies of Tan and colleagues10,17,18 characterized autoantibodies found in the sera of a small percentage of patients with systemic lupus erythematosus (SLE). These autoantibodies stained the nucleus of proliferating cells and reacted with an acidic polypeptide of molecular weight of 36 kDa19,20 that was termed proliferating cell nuclear antigen (PCNA). Later, Mathews and co-workers21 showed that cyclin and PCNA were identical, and Bravo et al.12 and Prelich et al.13 identified this polypeptide as the auxiliary protein of DNA polymerase δ.11
Immunofluorescence studies using cyclin (PCNA) autoantibodies carried out in our laboratory,3,22,23 as well as that of Bravo,9,24 showed that in methanol fixed cells only S phase cells reacted with the antibodies and that various patterns of cyclin (PCNA) antigen distribution subdivided S phase (Figure 4). Furthermore, many of the cyclin (PCNA) patterns were shown to mimic topographical patterns of DNA synthesis.3,9,23,25,26 In particular, late patterns of nucleolar DNA replication as determined by [3H]-thymidine autoradiography (see Figure 5B) could be superimposed with immunofluorescence pattern of cyclin (PCNA) antigen distribution (see Figure 5A).2 These observations, as well as others, argued strongly for a role of this protein in chromosomal DNA replication.
Given space limitations, we have listed in Table 1 relevant information concerning cyclin (PCNA) as entered in the comprehensive computerized database of cellular proteins from AMA cells.27 The role of cyclin (PCNA) in DNA replication is reviewed in the next article.
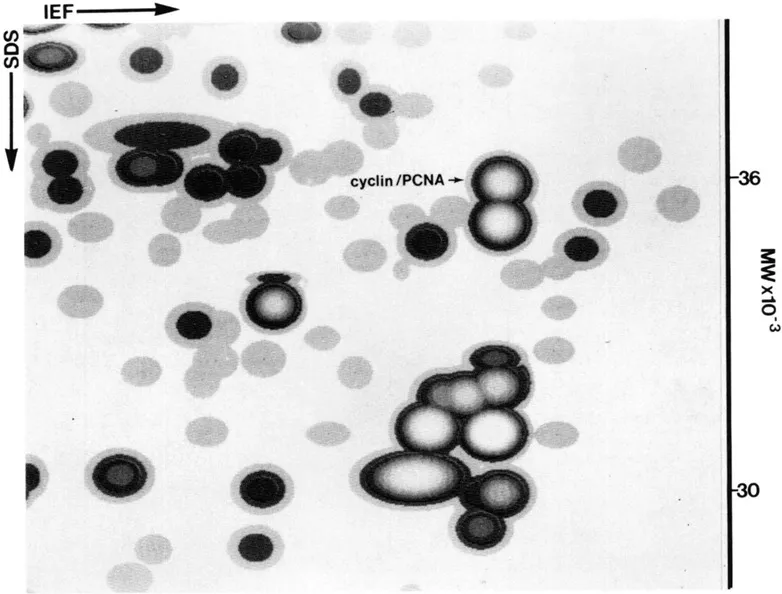
FIGURE 1. Synthetic image of a fraction of an IEF fluorogram of [35S]-methionine labeled proteins from transformed human amnion cells (AMA). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE, 15% running gel; 5% stacking gel) was carried out essential as described by Laemmli.28 Two dimensional gel electrophoresis (IEF) was carried out as described by Bravo et al.29 In short, the first dimension wa performed in 130 mm × 2 mm 4% w/v polyacrylamide gels containing 2% w/v carrier ampholytes (1.6% pH 5—7, Serva; 0.4% pH 3.5—10 LKB) (18 h at 400V). First dimension gels were equilibrated in 3 ml (3 min at room temperature) of equilibration solution (0.06 M Tris-HCL, pH 6.8, 2% SDS, 100 mM DTT and 10% glycerol).30 First dimension gels were applied to the second dimension with the aid of agarose solution (0.06 M Tris-HCL, pH 6.8, 2% SDS, 100 mM DTT, 10% glycerol, 1% agarose and 0.002% Bromophenol Blue).30 Following fluorography,31 the dried gels were exposed to X-ray films for various periods of time and scanned using an Eikonix series 78/99 digital scanner and PDQ-Scan software (Protein Database, Incorporated, N.Y.). Data was analyzed using the PDQUEST software (Protein Database, Incorporated)32 running on a MASSCOMP CPV 5520 computer.
Acknowledgments
We would like to thank S. Himmelstrup Jørgensen for typing the manuscript and O. Sønderskov for photography. This work was supported by grants from the Danish Biotechnology Programme, the Danish Cancer Society, the Danish Rheumatoid Society, NOVO, the Aarhus University Research Fund, and the Fund for Laegevidenskabens Fremme.
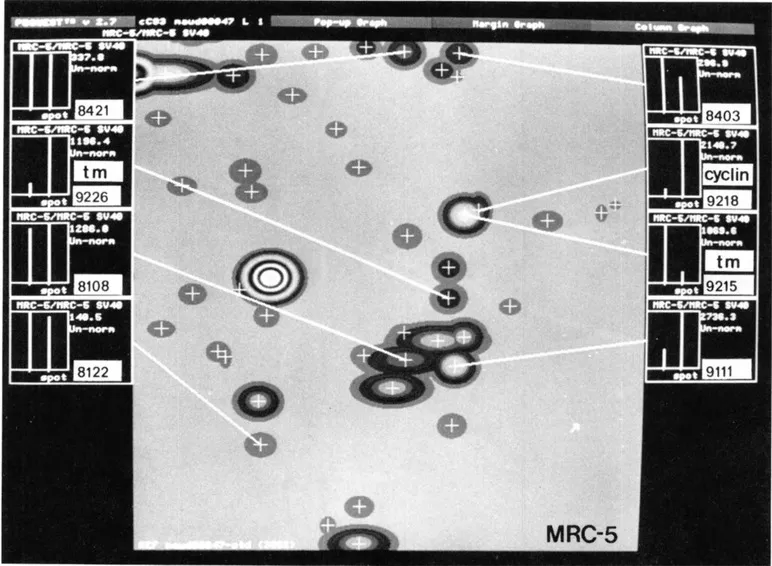
FIGURE 2. Synthetic image of a fraction of an IEF fluorogram of [35S]-methionine labeled proteins from normal MRC-5 fibroblasts. The histograms show levels of synthesis of a few proteins in MRC-5 (left bar) and MRC-5 V2 (right bar) fibroblasts. tm: tropomyosin. (From Celis, J. E., et al., Electrophoresis, 10, 76, 1989. With permission.)
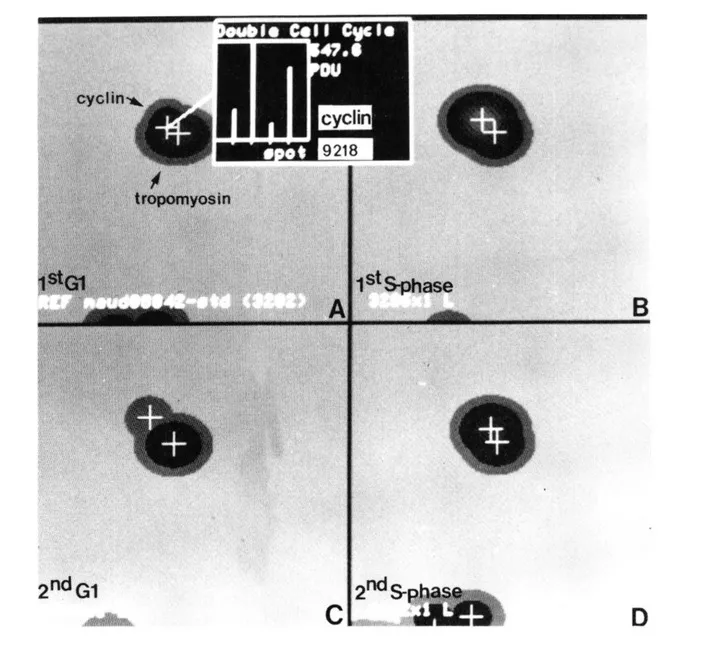
FIGURE 3. Synthesis of [35S]-methionine labeled cyclin (PCNA) during two successive cell cycles of AMA cells. Cells were labeled for 2 h. Synthetic images (IEF) from the following samples are shown: (A) G, cells (first G,) labeled 5 h after plating mitotic cells; (B) S phase cells (first S phase) labeled 14 h after plating mitotic cells; (C) G, cells (second G,) labeled 28 h after plating mitotic cells; (D) S phase cells (second S phase) labeled 35 h after plating mitotic cells. The height of the bars is scaled to the maximum detected value. Actual PDU (Protein Database Units) for cyclin (PCNA): (A) first G,, 172.4; (B) first S phase, 547; (C) second G1, 99.3; (D) second S phase, 487.3. (From Celis, J. E., et al., Electrophoresis, 10, 76, 1989. With permission.)
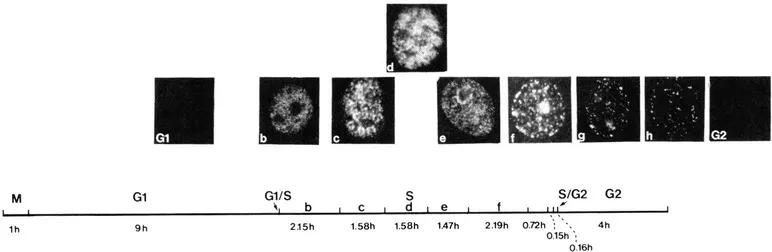
FIGURE 4. Sequence of cyclin (PCNA) antigen distribution during the cell cycle of AMA cells. Patterns of cyclin (PCNA) antigen distribution subdivide S phase (Sb-Sh).3,9,22,24 The duration of each phase is indicated in the figure.23 Mitotic, Gt and G2 cells contain cyclin (PCNA), but this form is not recognized by the autoantibodies in methanol fixed cells.3,26
TABLE 1
Entries for Cyclin (PCNA) in the Protein Database of Human AMA Cellsa
Entries for Cyclin (PCNA) in the Protein Database of Human AMA Cellsa

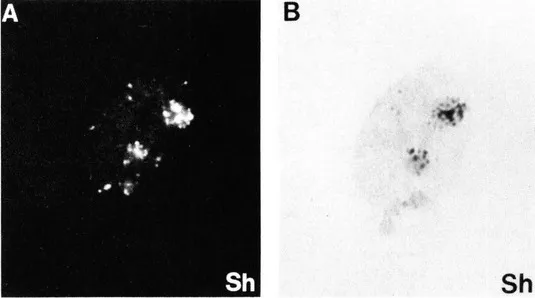
FIGURE 5. Immunofluorescence patterns of cyclin (PCNA) antigen distribution mimic topographical patterns of DNA replication. (A) immunofluorescence; (B) autoradiography ([methyl-3H]-thymidine incorporation) of BSCl cells depicting pattern Sh. (From Madsen, ...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Section 4: GROWTH REGULATION
- Section 5: DIFFERENTIATION, TISSUE INTERACTIONS
- Index