
eBook - ePub
The Melanotropic Peptides
Volume III: Mechanisms of Action and Biomedical Applications
This is a test
- 173 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
The three volumes on "The Melanotropic Peptides" are the outcome of a conference of the same name that was held in Tucson, Arizona, from October 11-12, 1986. Volume III discusses the known mechanisms of action of the two melanotropic peptides, and concludes with a discussion of the possible biomedical applications of the melanotropins.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access The Melanotropic Peptides by M.E. Hadley in PDF and/or ePUB format, as well as other popular books in Medicine & Alternative & Complementary Medicine. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
MELANOTROPIN BIOASSAYS
Mac E. Hadley and Ana Maria de L. Castrucci
TABLE OF CONTENTS
I. Introduction
A. The Melanophore Index (MI)
II. Frog Skin Bioassays
A. In Vitro Frog Skin Bioassay
B. In Vivo Frog Skin Bioassay
III. Lizard (Anous) Skin Bioassay
IV. Teleost (Fish) Skin Bioassay
V. Melanoma Bioassays
A. Melanoma Tyrosinase Bioassay
B. Melanoma Adenylate Cyclase Assay
C. Melanoma Clonogenic Assay
D. In Situ Melanin Assay
VI. Prolonged (Residual) Actions of Melanotropins
VII. Perspectives
Acknowledgments
References
I. Introduction
Bioassays have played an important role in determining the presence of hormones in the blood or other tissues as well as determining the biological potency of structural analogs of hormones. The frog skin bioassay, developed by Shizume et al. in 1954, has been particularly important in advancing our knowledge about the melanotropins. This bioassay, like the earlier assays for melanotropins, involved observations or recordings of the responses of pigment cells (chromatophores, particularly melanophores) present within the skin. Both in vitro as well as in vivo melanotropin bioassays have been employed in several vertebrate species. Melanoma cells provide a unique bioassay for determining the biochemical correlates of melanotropin action. In the present report some of the more commonly utilized bioassays for melanotropic peptides will be described. For a detailed discussion of the early bioassay systems for the melanotropins, several references are available,2-6 in addition to the classical monograph by Parker.7 For information on the radioimmunoassay of melanotropins several references are now available (see Wilson, Volume I, Chapter 11).
A. The Melanophore Index (MI)
The earliest studies of melanophore responses to melanotropins utilized a so-called “melanophore index” (MI).8 By this method the response of melanophores to a variety of stimuli could be subjectively quantitated by assigning numbers to the observed degree of melanosome (melanin granule) dispersion within the cells. In response to melanotropins, the perinuclearly aggregated (concentrated) melanosomes (MI-1) begin to disperse out into the dendritic processes of the melanophore until a maximum response (MI-5) is obtained (Figure 1). This assay is presently still used by many investigators throughout the world to study melanophore responses of teleosts, amphibians, and reptiles to melanotropic peptides. An analogous “chromatophore index” can be similarly utilized to study the response of other integumental chromatophore types (iridophores, erythrophores, and xanthophores) to melanotropins.
II. Frog Skin Bioassays
The skin of the frog has been most widely used for the study of the melanotropic activity of peptides. Both in vitro and in vivo bioassays have been utilized. Both assays involve the response of integumental chromatophores to stimulation by melanotropins. This response to exogenous melanotropins is identical to that resulting from the release of endogenous melanotropins from the pars intermedia of the pituitary gland. Melanotropins stimulate the dispersion of melanosomes out into the dendritic processes of melanophores and concomitantly stimulate the aggregation of reflecting platelets within iridophores. The composite structural unit of color change is referred to as the “chromatophore unit” (see Bagnara, Volume II, Chapter 2).
A. In Vitro Frog Skin Bioassay
Skins from the leopard frog, Rana pipiens, have been most commonly employed in the in vitro bioassay. However, skin from other ranid species as well as toads, appears to be equally useful. The bullfrog, R. catesbeiana, has a greater worldwide availability and, like R. pipiens, is very sensitive to melanotropins. In the present report, the details provided are equally applicable to both species of frog. The real advantage of R. catesbeiana is that the frogs are generally larger than other ranids, and most of the dorsal body skin can also be utilized in addition to skin from the legs.
Frogs are sacrificed by decapitation and/or pithing, and the leg and the thigh skins are removed from the animals and placed in a physiological saline (Ringer) solution. The skins are then mounted on metal rings and held in place by an outer plastic ring as described for the original bioassay.1 The mounted skins are then placed in beakers containing Ringer solution. The addition of a melanotropin to the bathing solution results in darkening of the skins, and subsequent removal of the peptide by placing the skins in Ringer in the absence of the peptide results in a relightening of the skins. Changes in skin color (reflectance) are monitored by a Photovolt reflectometer and recorded as percent changes from the initial base (zero) value. In three species of ranids studied α-melanocyte-stimulating hormone (α-MSH) was found to be slightly (2—4 fold) more potent than (β-MSH.2 The minimal effective dose found to significantly darken the skins above control levels is about 2 to 4 × 10−11 M (Figure 2). A maximal response is obtained at about 60 min following addition of a melanotropin, and the skins are light again by 90 to 120 min following removal of the peptides (Figure 3). Therefore, skins can be used several times in one day. All melanotropin analogs studied to date have provided parallel dose response curves (see Cody et al.,9 Chapter 7, this Volume).
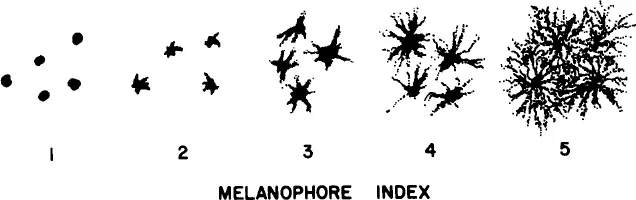
FIGURE 1 Melanophore index (MI). Melanotropins stimulate melanosome dispersion; melanosomes migrate from a perinuclear position within the melanophore (MI-1) out into the dendritic processes of the pigment cell (MI-5).
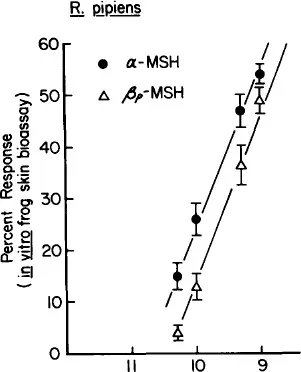
FIGURE 2 In vitro frog (R. pipiens) skin bioassay. Four-point dose-response curves to α- and [β-MSH. All points are significantly different (p <0.05) from the control set of skins, (n = 16 per point).
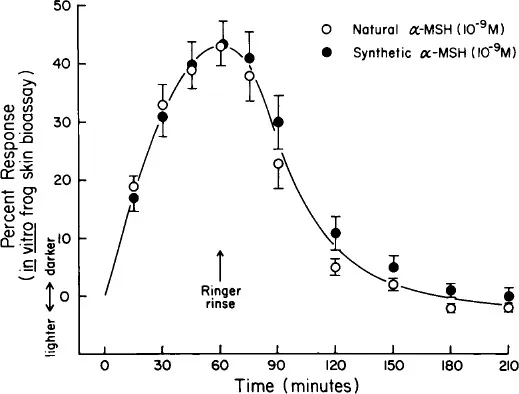
FIGURE 3 Reversal of the darkening response of frog skins to natural and synthetic α-MSH, after transfer of the skins to Ringer in the absence of the melanotropins (n = 6).
B. In Vivo Frog Skin Bioassay
We recently described an in vivo bioassay for melanotropins using the frog, R. pipiens.2 The reflectance method was again used, and we determined the in vivo potencies of α- and β-MSH (Figure 4A and B). Unexpectedly, the melanotropins were as active in the in vivo assay as in the in vitro bioassay. The frogs darkened in a dose-response manner to the injected melanotropins. α-MSH as low as 5 × 10−11 mmol/10 g/frog induced significant darkening of the animals. Both α- and β-MSH, but particularly α-MSH, are rapidly inactivated in frog serum in vitro (see Castrucci and Hadley, Chapter 1, this volume). Therefore, it was surprising that an injection of about 5 × 10−10 mmol/10 g body weight of α-MSH gave a response approximately equal to 10−9 mmol of peptide/10 mℓ of bathing medium in vitro.
Kastin et al.10 used hypophysectomized R. pipiens and found by using the classical MI that the in vivo assay was as sensitive as the in vitro assay for the determination of the potency of a number of adrenocorticotropin (ACTH)-like peptides. These observations are even more surprising since these peptides unlike α-MSH, which is N-terminal acetylated and C-terminal amidated, should be even more vulnerable to inactivation by exopeptidases. In this regard, one might even expect that β-MSH, although somewhat longer than α-MSH, would be more susceptible than the shorter α-MSH to inactivation by exopeptidases. The actions of β-MSH in vivo are more prolonged than an equimolar concentration of α-MSH when injected into the frog (Figure 4 A and B). The more prolonged actions of β-MSH may relate to the fact that the peptide is less susceptible to inactivation by serum enzymes than is α-MSH.
III. Lizard (Anous) Skin Bioassay
The American anole (Anolis carolinensis), the so-called “American chameleon”, is noted for its ability to rapidly change from a very light green color to a very dark brown color. Small pieces of Anolis skin can be floated on Ringer solution wherein they are light green in color; when melanotropins are added to the bathing solution the skins change to an olive green color and then to a light tan color, to a brown color, and finally to a dark brown color. These changes can conveniently be numerically ranked as in the classical MI from one (ligh...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Content Page
- Preface
- The Editor
- Contributors
- Chapter 1 Melanotropin Bioassays
- Chapter 2 Melanotropin Mechanisms of Action: Melanosome Movements
- Chapter 3 The Role of Protein Phosphorylation in the Mechanism of Action of α-Msh
- Chapter 4 Melanotropin Mechanisms of Action: Melanogenesis
- Chapter 5 Melanotropin Receptors and Signal Transduction
- Chapter 6 Cyclic, Conformationally Constrained Melanotropin Analogs: Structure-Function and Conformational Relationships
- Chapter 7 Melanotropin Three-Dimensional Structural Studies by Physical Methods and Computer-Assisted Molecular Modeling
- Chapter 8 Melanotropin Receptors: Studies with Labeled Melanotropins
- Chapter 9 Melanotropic Peptides: Biomedical Applications
- Chapter 10 The Melanotropic Peptides: A Summary
- Index