Chapter 1
The Modern Pharmaceutical Development Challenge: BCS Class II and IV Drugs
Gregory K. Webster,a Robert G. Bell,b and J. Derek Jacksonc
aAbbVie Inc., Global Research and Development, 1 N. Waukegan Rd., North Chicago, IL 60064, USAbDrug & Biotechnology Development, LLC, 406 South Arcturas Avenue, Suite 5, Clearwater, FL 33765, USAcFlexion Therapeutics, Inc., 10 Mall Road, Suite 301, Burlington, MA 01803, USA [email protected],
[email protected],
[email protected] 1.1 Introduction
Since the 1960s, pharmaceutical companies have been charged with monitoring the characteristics of their oral dosage forms dissolving in controlled media. Early dissolution testing focused on the quality control of the dosage form manufacturing. Dissolution testing provided unique capabilities in monitoring integrated production parameters that affect the dissolution rate: tablet hardness, excipient control, particle size, etc. The technique became required for the routine testing of oral dosage forms worldwide. The early history of dissolution testing is well documented in the literature.1, 2 and 3
Poorly Soluble Drugs: Dissolution and Drug Release
Edited by Gregory K. Webster, J. Derek Jackson, and Robert G. Bell
Copyright © 2017 Pan Stanford Publishing Pte. Ltd.
ISBN 978-981-4745-45-1 (Hardcover), 978-981-4745-46-8 (eBook)
www.panstanford.com
The additional advantage of early dissolution testing was the relative simplicity of the active pharmaceutical ingredients (API) and their associated dosage forms. These soluble drugs were readily bioavailable as they tended to be both highly soluble and highly permeable in the gastrointestinal tract. Thus, for highly soluble and permeable drugs, dissolution assessment was adequate to ensure clinical performance through simple standardized solutions such as water and acidic media. Discrimination focused primarily on the disintegration and dispersion from solid oral dosage forms with the active ingredients behaving predictably. Under these conditions, the dissolution test was effective in predicting performance in human clinical trials and was denoted as a clinically relevant method. With further evolution in drug formulation technologies, dissolution testing harmonized on the four basic apparatus still in use today and commonly referred to by their United States Pharmacopeia (USP) designations of apparatus 1, apparatus 2, apparatus 3, and apparatus 4.4 Other USP apparatus designations, such as apparatus 7, tend to be for non-oral dosage forms.
Today, dissolution testing has broadened its applications and scope in moving forward from a simple quality control test to use in predicting in vivo/in vitro correlations (IVIVC) for soluble and permeable dosage forms and clinical relevance for many others. The simple formulations found in the developing years of dissolution testing have given way to more complex technologies to advance the bioavailability of less soluble molecules. The goal of this book is to move past the existing dissolution texts referenced earlier, which primarily denotes dissolution testing for soluble drugs, and to focus on the issues of dissolution testing current with the molecules in development today. This text builds upon the solid foundation of the earlier works1, 2 and 3 to the current application of dissolution and drug release technologies with an emphasis on poorly soluble drugs.
1.2 Changing Drug Emphasis
The dissolution platforms of USP apparatus 1–4 were key technologies in facilitating soluble drugs to market. The techniques, instruments, and the simple buffer systems used ideally characterized the dosage forms being developed. However, as the active pharmaceutical ingredients (API) became increasingly more complex (in terms of solubility and permeation in the gastrointestinal tract), these simple drug release mechanisms did not correlate. The Biopharmaceutics Classification System was developed by Amidon et al.6 and published as a guidance by the U.S. Food and Drug Administration (FDA) for predicting the intestinal drug absorption of oral dosage forms.7 The BCS system has become the gold standard to categorize and estimate oral drug absorption based on the drug’s solubility and intestinal permeability characteristics.
1.2.1 BCS Classification System
The BCS categorization of drugs is based on the premise that as a drug dissolves, this concentration is available to move across the membrane and correlate to intestinal absorption. Gastric solubility is established through in vitro chemical testing at various conditions and pH’s representative of the human gastrointestinal tract. The permeability of the drug is based on initial lipophilicity testing and further studied in animal models, tissue studies, cultured epithelial cells such as Caco-2 testing, and ultimately in humans (mass balance, absolute bioavailability intestinal perfusion testing, etc.).
With the drug solubility and permeability established, the BCS system segregates drugs into four classes, as illustrated in Table 1.1.
Per the FDA Guidance, the target drug is deemed “highly soluble” when the highest dose strength is soluble in <250 mL of aqueous media over a pH range of 1 to 7.5. The drug is deemed “highly permeable” if the extent of absorption in humans is determined to be > 90% of an administered dose. In addition, a drug formulation is deemed “rapidly dissolving” when > 85% of the labeled amount of drug substance dissolves within 30 minutes using USP apparatus 1 or 2 and a volume of ⩽500 mL of buffered media.
Table 1.1 BCS drug classification
| High solubility | Low solubility |
High permeability | Class 1 High solubility High permeability | Class 2 Low solubility High permeability |
Low permeability | Class 3 High solubility Low permeability | Class 4 Low solubility Low permeability |
Knowing the drug’s BSC category allows the pharmaceutical scientist to evaluate the rate-limiting step in the absorption of the drug. For class 1 drugs the high solubility and high permeability of the API indicate that the absorption in the gastrointestinal tract should be dissolution rate limited. For class 2 drugs, because the drug is less soluble but still very permeable, this class of drugs should also be dissolution rate limited. With the solubility being high in class 3 but permeability of the drug low, the API in this class becomes absorption rate limited. For this class of drugs, dissolution is seldom clinically relevant. The FDA has recently issued a guideline denoting that, under special cases, a class 3 drug can be addressed with class 1 specifications for biowaiver studies.8 However, this guidance does not propose that class 3 drugs can readily achieve IVIVC via traditional dissolution testing. IVIVC for class 1 and 3 drug products is not likely unless drug dissolution is significantly slowed due to formulation (e.g., MR formulation) or the compound is borderline BCS 1 with respect to solubility. This is acknowledged in the FDA Guidance for Industry for Dissolution Testing of IR solid oral dosage forms.9 Class 4 drugs rely upon transporters and other biological means to transport across the membrane. Dissolution may be able to characterize this transport, but as with BCS class 3 drugs, dissolution is challenged to characterize the absorption of these drugs.
1.2.2 Poorly Soluble Drugs
A main focus of this book is to focus on the dissolution and drug release of BCS class 2 drugs. These drugs are permeable but with limited solubility. As of 2006, BCS class 2 drugs made up approximately a third of the global pharmaceutical market.10 As will be illustrated in Chapter 5, formulation technologies have gone a long way to increasing the bioavailability of these molecules. Typically, these formulations are often working with amorphous or nanoparticle material technologies.
1.3 The Dissolution Market
Dissolution is a significant technology found in the laboratories of every major pharmaceutical business concern with oral dosage forms and predominately uses chromatography and spectroscopy for final quantitative analysis. As such, perhaps it is time that dissolution testing warrants a chapter in undergraduate instrumental analysis textbooks. In 2009, approximately 3400 instruments were sold with a market expecting to grow at 8% annually.11 Today this market is valued at over $150 million and tied directly to the pharmaceuticals market. The pharmaceutical industry accounts for approximately 75% of these sales. The remaining 25% is split between contract research organizations, biotech, academia and agriculture.
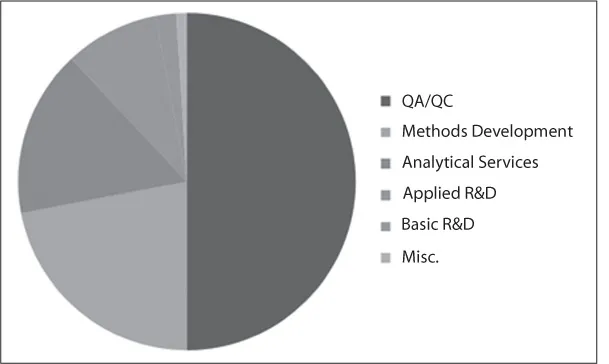
As seen in Fig. 1.1, quality control testing dominates the demand for dissolution testing.
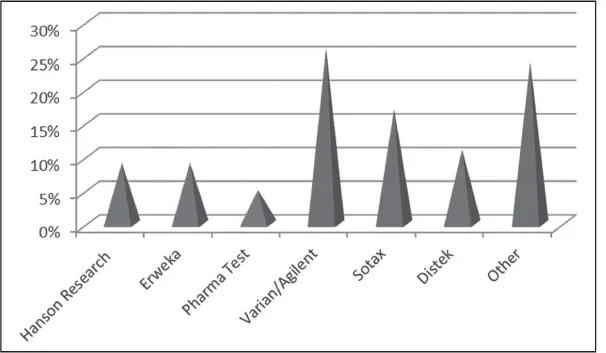
As Fig. 1.2 shows, in 2009, the largest dissolution vendor was Varian/Agilent. The market was diversified with several vendors; however, even with this diversity, the technique still today revolves around the standard USP designations of apparatus 1 and 2, with less significant portion of the market operating apparatus 3 and 4. However, as will be seen in Chapters 8 and 9, these later instrument technologies may play a more significant role in the dissolution and drug release testing in the years to come.
1.4 Dissolution and Drug Release in the Pharmaceutical Industry
The goal of this text is for leading scientists in the field to discuss the current applications of dissolution and drug release to poorly soluble molecules. Each chapter provides a current view from leading practitioners of the material presented. While much of the instrumentation of dissolution and drug release has not changed dramatically from the traditional USP apparatus, the approaches and applications have moved forward with the changing molecules being brought to market. This book builds upon the technique from its initial and early developments. Details on the development and theory of dissolution and drug release can be found in the literature.1, 2 and 3
1.4.1 Solubility Determinations for Pharmaceutical API
Aqueous solubility for the active pharmaceutical ingredient (API) in the final formulation is a significant factor that influences the pharmacokinetic profile of a drug. There are various analytical methodologies and computational models for the prediction of solubility of the API. The solubility in the body can often be different from that determined in common in vitro buffer systems that are typically used for pharmaceutical quality control processes. The quality of computational models is also affected by the accuracy of the experimental solubility data. High-throughput discovery processes have driven the development of high throughput screening processes for measurement of physicochemical determinations including lipohilicity, pKa and solubility, but the accuracy of these is often compromised by the requirement for speed and the use of non-biorelevant media in the determination. Kofi Asare-Addo and Barbara R. Conway from the University of Huddersfield will discuss the nuances regarding the solubility determinations for APIs.
1.4.2 Use of Surfactants in Dissolution Testing
Before any relevant dissolution or drug release testing can occur in formulated product, the analytical target must be dissolved in the test matrix. In dissolution testing, this is often ...