
This is a test
- 208 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
This text comprises of two volumes discussing the regulation of carbohydrate metabolism.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Regulation Of Carbohydrate Metabolism by Rivka Beitner in PDF and/or ePUB format, as well as other popular books in Medicine & Alternative & Complementary Medicine. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
GLYCOLYTIC ENZYME ORGANIZATION VIA THE CYTOSKELETON AND ITS ROLE IN METABOLIC REGULATION
TABLE OF CONTENTS
I. | Historical Perspective | |
II. | The Nature of the Adsorbent | |
A. | Muscle: The Role of Actin and the I-Band | |
B. | The Role of Tropomyosin (TM) and Troponin (TN) | |
C. | The Specificity of Enzyme Binding Sites | |
D. | Piggy-Back or Indirect Binding and Glycolytic Enzyme Organization | |
E. | Nonmuscle Cells and Cytoskeletal Actin | |
III. | Dynamics of Enzyme Organization | |
A. | Metabolic Dependence of Enzyme Binding | |
B. | Effectors of Binding | |
C. | Genetic Determinants of Binding | |
IV. | Functional Significance of Binding | |
A. | Influence on Catalytic Expression | |
B. | Structural Considerations | |
References | ||
I. HISTORICAL PERSPECTIVE
While the classification of the glycolytic enzymes as soluble is an operational definition which reflects the relative ease with which these proteins are extracted from cells and tissues, the term also has the conceptual connotation that these enzymes exist free in solution within the cell. Unfortunately this implied intracellular distribution is still widely held despite the wealth of accumulated evidence that these enzymes are represented in both the particulate and soluble phases of the cell.
Many of the early reports 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 of glycolytic enzyme associations with cell particulate fractions occurred at a time when investigations of organelles as multienzyme complexes were at their height.15 The observation of glycolytic enzymes bound to subcellular structures naturally led speculation in the direction of the existence of a discrete glycolytic complex. This viewpoint was encouraged by the demonstration by Green et al.16 of the isolation of membranous components from both red cell and yeast which were capable of catalyzing the complete glycolytic sequence. Whatever expectations there were for the existence of a complete glycolytic complex, the notion was grievously injured by de Duve in 1972 who compared the sedimentation of some glycolytic enzymes in a “cell sap” (in fact a conventional supernatant fraction) of liver with that of the purified enzymes and found that they sedimented identically and so concluded that complex formation between these enzymes in the cytoplasm did not occur.17 When this was coupled with a recognition17 that most of the in vitro studies of glycolytic enzyme binding to subcellular structures have been performed only under very low ionic strength conditions,17 then it has been convenient to dismiss not only the notion of glycolytic enzyme complexes but also enzyme binding to structural components as nonspecific and irrelevant.
The soluble fraction (cell sap) of classical subcellular fractionation procedures18 cannot be equated with the cytoplasm of the cell even with due attention to the ionic strength. It is now acknowledged that the cytoplasm of eukaryotic cells is a highly concentrated, thixotropic protein solution with a high degree of structure imposed by the all-pervasive cytoskeletal network formed by the actin, microtubule, and intermediate filament systems.19, 20, 21, 22, 23 Indeed it has been the extensive studies of this cytoskeletal apparatus over the last decade24,25,26,27,28,29,30,31,32,33 that have finally brought about general acceptance of this view of the cytoplasm — a view it should be remembered which has been held by a number of workers long before the modern concepts of the cytoskeleton.34,35,36,37 There is no more telling testimony to the nature and structure of the cytoplasmic environment than to view the recent electron micrographs provided by Porter,38, 39 Heuser and Kirschner,40 and others.41,42 Keeping pace with these developments is the clear demonstration that interactions of glycolytic enzymes with cytoskeletal components can and do occur within this cellular environment.23,43, 44, 45, 46, 47, 48, 49
Despite these advances, however, we still lack a clear perspective as to the purposes served by enzyme binding. In skeletal muscle, for example, of the ten enzymes which catalyze the conversion of glucose-6-phosphate to lactate, at least four, aldolase (ALD), phosphofructokinase (PFK), glyceraldehyde-3-phosphate dehydrogenase (GPDH), and pyruvate kinase (PK) are capable of binding very strongly to actin-containing filaments, while the other six bind to a lesser but no less appreciable degree.22,23 This binding varies between different types of muscle and even within the same muscle in response to different physiological stimuli (Table 1). This level of complexity in just one type of tissue serves to obscure both the relevance and role of enzyme binding in the glycolytic economy.
In this article we seek to establish the principles involved in glycolytic enzyme binding and then to consider the metabolic and structural purposes served by the interaction of these enzymes with the cytoskeletal apparatus of the cell.
Table 1
GLYCOLYTIC ENZYME BINDING LEVELS IN DIFFERENT MUSCLESa
GLYCOLYTIC ENZYME BINDING LEVELS IN DIFFERENT MUSCLESa
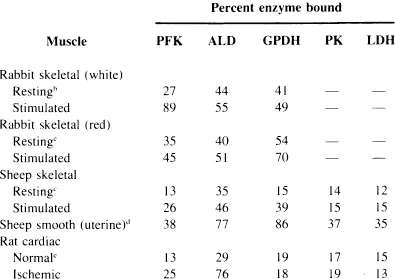
a Enzyme binding as determined by Clarke, F. M., Morton, D. J., and Shaw, F., Biochem. J., 186, 104, 1980 (Reference 97).
b Enzyme binding as determined by Walsh, T. P., Clarke, F. M., and Morton, D. J., unpublished data (Reference 144).
c Enzyme binding as determined by Walsh, T. P., Masters, C. J., Morton, D. J., and Clarke, F. M., Biochem. Biophys. Acta, 675, 29, 1981 (Reference 98).
d Enzyme binding as determined by Clarke, F. M., Morton, D. J., Hamilton, D., and Huxham, G., unpublished data (Reference 145).
e Enzyme binding as determined by Clarke, F. M., Stephan, P., Huxham, G., Hamilton, D., and Morton, D. J., Eur. J. Biochem., 138, 643, 1984 (Reference 142).
II. THE NATURE OF THE ADSORBENT
A. Muscle: The Role of Actin and the I-Band
In skeletal muscle, a proportion of the enzymes of glycolysis are discretely localized on the structural framework provided by the actin-containing filaments of the contractile apparatus. The physiological reality of this binding was established principally by Pette and co-workers. Following on the original observation of Bucher50 that a significant portion of the activity of many glycolytic enzymes was not readily extractable from muscle, Pette and his colleagues applied a series of histochemical,43,51 immunofluorescent,44 and biochemical techniques52, 53, 54 to show that most of the glycolytic enzymes are localized within the I-band of muscle fibers.
The histochemical and immunofluorescent studies revealed that phosphorylase, phosphoglucomutase, glucose phosphate isomerase, phosphofructokinase (PFK), triosephosphate isomerase (TPI), glyceraldehyde-3-phosphate dehydrogenase (GPDH), pyruvate kinase (PK), and lactate dehydrogenase (LDH) were all discretely localized within the I-band, whereas hexokinase (HK) showed a distribution in keeping with its known association with mitochondria.55 While these cytological studies by themselves cannot unambiguously distinguish between a localization in the interfilamentary space or the overlying sarcoplasmic reticulum, Pette and others have adduced a number of arguments to support their conclusion that the glycogenolytic and glycolytic enzymes are localized in the region of, or directly associated with the thin filaments of the contractile apparatus. Arnold and Pette found that many of these enzymes interact quite strongly with F-actin, the major structural protein of the I-band thin filaments.52, 53, 54 This was confirmed by Clarke and Masters who establi...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Chapter 1 Glycolytic Enzyme Organization Via the Cytoskeleton and Its Role in Metabolic Regulation
- Chapter 2 Regulation of Glycogen Metabolism
- Chapter 3 Effects of the Abnormal Carbohydrate Metabolism Present in Glycogen Storage Disease on Intermediary Amino Acid and Lipid Metabolism
- Chapter 4 Effect of Ethanol on Carbohydrate Metabolism
- Chapter 5 Effect of Sucrose and Fructose on Carbohydrate and Lipid Metabolism and the Resulting Consequences
- Chapter 6 New Perspectives on Carbohydrate Metabolism in Tumor Cells
- Chapter 7 Insulin Binding and Metabolism by Hepatocytes in Primary Culture
- Index