
This is a test
- 443 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Organic Sulfur Chemistry
Book details
Book preview
Table of contents
Citations
About This Book
This volume contains fundamental knowledge regarding the structure and mechanisms of organic sulfur chemistry. Topics include sulfur bondings, effects of sulfur groups, stereochemistry around sulfur, substitution, ligand coupling within s-sulfurane, oxidation, reduction and rearrangement. References in this work total over 2, 300. Anyone with an interest in organic sulfur chemistry will find this book to be fascinating reading.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Organic Sulfur Chemistry by Shigeru Oae in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Chapter 1
SULFUR BONDING
I. INTRODUCTION
Sulfur lies in the second row of the periodic table and belongs to the oxygen family. Therefore, the familiar functional groups — the alcohols, ethers, ketones, and peroxides — have their counterparts in the sulfur series — mercaptans (thiols), sulfides (thioethers), thioketones, and disulfides — and the next member of the family, selenium, is found in selenols, selenides, selenoketones, and diselenides.
All those divalent species have similar physicochemical properties. However, there are several fundamental differences. For example, no stable compound having a polyoxide linkage (–On–) is known, whereas there are numerous sulfur compounds bearing polysulfide chains. While three coordinate oxygen compounds such as oxonium ions are rather unstable and only a few have been isolated, corresponding sulfur analogues, such as sulfoxides, sulfilimines, and sulfonium ylides, are quite stable. Sulfur can form four coordinate species, such as sulfones, sulfoximines, and even hexacoordinate compounds such as SF6 in stable forms. Selenium also can form similarly polycoordinated species. However, they are somewhat less stable than the corresponding sulfur analogues, presumably due mainly to the larger size of the atom.
At one time, overlap between 2p orbitals to form a π-bond between carbon and oxygen atoms was believed to be greater than that between 2p and 3p orbitals to form a π-bond between carbon and sulfur atoms.1,2 However, this has been a controversial issue. The stable nature of polysulfide linkages as compared with that of polyoxide bonds, and the stabilities of polycoordinated sulfur species, such as sulfones, sulfoximines, SF4, or even SF6, were considered earlier to be due to the availability of energetically readily accessible 3d orbitals of sulfur atom. Although the original concept of hybrid of 3sp3d orbitals, suggested by Pauling for SF4,3 was replaced by the concept of hypervalency,4 the unusual stabilities of many polycoordinated sulfur compounds are still believed to be due to the added orbital interaction with readily accessible 3d orbitals.
An early MO calculation by Longuett-Higgins suggested the importance of 3d orbitals in the stabilization of thiophene,5 while Moffitt emphasized the symmetry of the contribution of 3d orbitals into hybridization of orbitals.6 However, it was the simple concept of atomic orbitals, first postulated by Kimball7 and later developed by Craig et al.,8 that has given the earlier fundamental basis for understanding the nature of sulfur bonding using 3d orbitals of sulfur. Accordingly, a brief discussion of this concept would be worthwhile.
There are five 3d orbitals for any of the second row elements such as sulfur, as shown in Figure 1. All these 3d orbitals are so diffused that it is not easy for any of these orbitals to overlap with other orbitals, e.g., 2p orbitals of the first row elements, such as carbon and oxygen. However, when the central sulfur atom bears a positive charge as the result of bonding to electronegative ligands, the 3d orbitals contract and are capable of overlapping with 2p orbitals of connecting adjacent atoms. Therefore, the central sulfur atom in the partially positively charged sulfoxide, sulfone, and sulfonium ion in π-bond formation is considered to be stabilized by the contribution of an additional interaction with one of the 3d orbitals. Among the five 3d orbitals, the first three may be used for π-bond formation, while the remaining two are mainly mobilized for the formation of σ-bonds.
As Figure 1 illustrates, there are four diffused lobes for each of these 3d orbitals and hence, the effective overlap between any 2p or 3p orbitals of adjacent atoms with 3d orbitals does not require any rigorous angular arrangement as in the effective overlap between 2p or 3p orbitals. This simple concept has been quite useful for understanding many important physicochemical properties and reactions of sulfur-centered organic compounds.
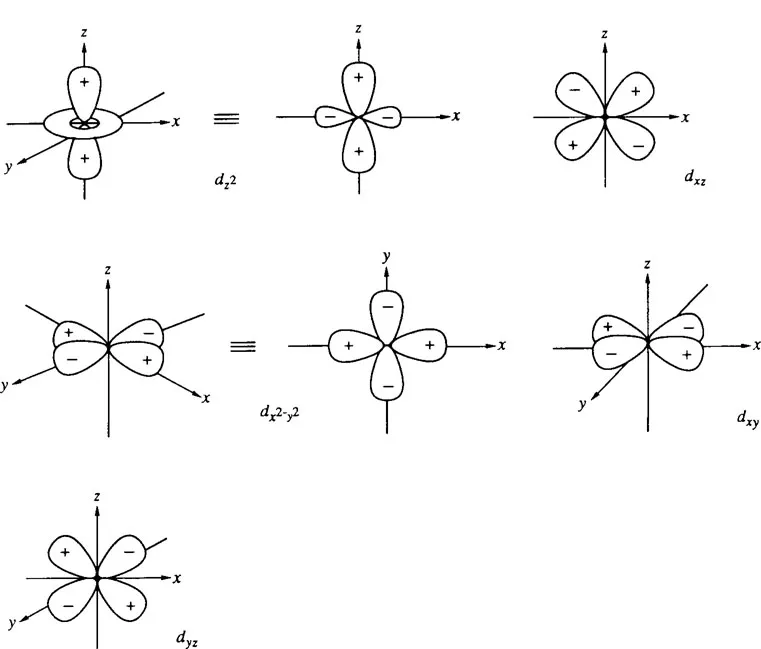
FIGURE 1. 3d orbitals.
However, during the late 1960s and early 1970s, this simple theoretical model and simple Slater-type MO calculation (STO), which take participation of 3d orbitals into consideration, were gradually replaced by ab initio calculations, which refute the importance of 3d orbital participation for resonance. Thus, the significance of ...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Chapter 1 Sulfur Bonding
- Chapter 2 The Stereoelectronic Effects of Sulfur Groups
- Chapter 3 Stereochemistry
- Chapter 4 Substitution
- Chapter 5 Ligand Coupling Reactions Within Hypervalent Species
- Chapter 6 Oxidation and Oxygenation
- Chapter 7 Reduction
- Chapter 8 Rearrangements
- Index