
This is a test
- 402 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
This intriguing and accessible book examines the experiments on neutrino oscillations. It argues that this history gives us good reason to believe in the existence of neutrinos, a particle that interacts so weakly with matter that its interaction length is measured in light years of lead. Yet, the scientific process has provided evidence of the elusive neutrino. Written in a style accessible to any reader with a college education in physics, Are There Really Neutrinos? is of interest to students and researchers alike. This second edition contains a new epilogue highlighting the new developments in neutrino physics over the past 20 years.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Are There Really Neutrinos? by Allan D. Franklin, Alysia D. Marino in PDF and/or ePUB format, as well as other popular books in Ciencias físicas & Astronomía y astrofísica. We have over one million books available in our catalogue for you to explore.
Information
1
The Road to the Neutrino
Radioactivity, the spontaneous transformation of one element into another, produces a particles (positively charged helium nuclei), or β particles (electrons), or γ rays (high-energy electromagnetic radiation). Experimental work on the energy of the electrons emitted in β decay began in the early twentieth century, and the observations posed a problem. If β decay were a two-body process (for example, neutron decays to proton + electron, or n → p + e), then applying the laws of conservation of energy and conservation of momentum requires that the electron emitted be mono energetic. Thus the observation that electrons were emitted with all energies from zero up to a maximal energy that depended on the radioactive element—a continuous energy spectrum—cast doubt on both of these conservation laws. Physicists speculated that perhaps the electrons lost energy in escaping the substance, with different electrons losing different amounts of energy, thus accounting for the continuous energy spectrum. Careful experiments showed that this was not the case, so the problem remained. In the early 1930s Wolfgang Pauli suggested that a low-mass neutral particle, named by Enrico Fermi the neutrino, was also emitted in β decay. This solved the problem of the continuous energy spectrum because in a three-body decay (neutron → proton + electron + neutrino) the energy of the electron was no longer required to be unique. The electron could have a continuous energy spectrum, and the conservation laws were saved.
The story told above has several virtues. It is clear and seems to have an almost inevitable logic. Physicists proceeded from the observation of a continuous energy spectrum in decay, via the application of the conservation laws of energy and momentum, to the need for a new, low-mass, neutral particle that was also emitted in β decay. The only problem with this story is that it is incomplete. The actual history is both far more complex and far more interesting. The first difficulty with the story concerns the observation of the continuous energy spectrum in β decay. In this chapter I will show how physicists came to the conclusion that the spectrum is continuous—a process that took some 30 years. It was not as simple as just measuring the energy of the electrons emitted in β decay.
The story begins with the discovery of radioactivity, the process in which an atomic nucleus emits a particle and is transformed into the nucleus of another element. I will discuss how physicists found that one of the types of particles emitted, the rays, was an electron. Early work on the energy spectrum of the emitted electrons indicated that the spectrum consisted of groups of electrons with different discrete energies—a line spectrum. It was ultimately found that these lines were a real effect but were in fact a rather small effect on a larger continuous spectrum. The continuous spectrum was not accepted until it was shown that the electrons were not emitted with discrete energies that somehow lost energy in the emission process.
A The Discovery of Radioactivity
Our story begins in 1896 with the almost accidental discovery of radioactivity by Henri Becquerel (1896a, b, c, d, e). Becquerel’s work was stimulated by the then recent discovery of x rays by Wilhelm Rontgen in 1895. Becquerel had been working on phosphorescence, the delayed emission of light by a substance after it has been exposed to an external source of light. Becquerel was continuing in a family tradition. Both his father and his grandfather had worked in the field. After Rontgen s announcement, Becquerel began investigating whether phosphorescent substances would emit x rays if they were exposed to intense light. His initial experiments produced no effects, but when he used uranium salts, which he had prepared for phosphorescence experiments 15 years earlier, he found a striking effect. He described his experiment as follows:
One wraps a photographic plate … in two sheets of very thick black paper … so that the plate does not fog during a day’s exposure to sunlight. A plate of the phosphorescent substance is laid above the paper on the outside and the whole is exposed to the sun for several hours. When the photographic plate is subsequently developed, one observes the silhouette of the phosphorescent substance, appearing in black on the negative [Figure 1.1]. If a coin, or a sheet of metal… is placed between the phosphorescent material and the paper, then the image of these objects can be seen to appear on the negative. (Becquerel 1896a, translated in Pais 1986, pp. 45–46)
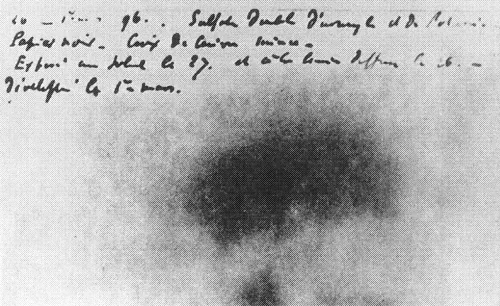
Looking at the plate in the figure, one sees a dark smudge—not very convincing evidence for anything. For Becquerel, however, it stimulated further investigation. He also performed the experiment with a piece of glass inserted between the phosphorescent substance and the black paper, which he noted “excludes the possibility of a chemical action resulting from vapors that might emanate from the substance when heated by the sun’s rays.” Having eliminated a plausible background effect that might have produced his observed effect, Becquerel concluded that “the phosphorescent substance in question emits radiations which penetrate paper that is opaque to light.”
One week later, Becquerel admitted that his earlier interpretation of his result was wrong. He published a paper demonstrating that his observed phenomenon had nothing to do with phosphorescence (1896b). William Crookes, a British physicist who often worked with Becquerel in his laboratory, described the discovery.
The writer visited Henri Becquerel’s laboratory one memorable morning when experiments were in progress which culminated in the discovery of the “Becquerel Rays” and of “Spontaneous Radioactivity.” Uranium salts of all kinds were seen in glass cells, inverted on photographic plates enclosed in black paper, and also the resulting images automatically impressed on the sensitive plates. Becquerel was working on the phosphorescence of uranium compounds after insolation [exposure to sunlight]; starting with the discovery that sun-excited uranium nitrate gave out rays capable of penetrating opaque paper [his earlier result] and then acting photographically, he had devised another experiment in which, between the plate and the uranium salt, he interposed a sheet of black paper and a small cross of thin copper. On bringing the apparatus into daylight the sun had gone in, so it was put back into the dark cupboard and there left for another opportunity of insolation. But the sun persistently kept behind clouds for several days, and, tired of waiting (or with the unconscious prevision of genius), Becquerel developed the plate. To his astonishment, instead of a blank, as expected, the plate had darkened under the uranium as strongly as if the uranium had been previously exposed to sunlight, the image of the copper cross shining out white against the black background. (Crookes 1909, p. xxii)
Becquerel observed the same effect with several uranium salts, from which he inferred that the effect was due to the presence of uranium. He confirmed this idea in an experiment in which he used only pure uranium metal and obtained the same result. He concluded that uranium was emitting a form of radiation that could both penetrate opaque paper and expose a photographic plate. Becquerel drew no conclusions about the nature of the radiation emitted, but he speculated that it might be some form of invisible phosphorescent radiation. He noted that although the existing evidence was consistent with such a hypothesis, he had not proved it. Subsequent experiments, by the Curies and others, showed that other substances, including the newly discovered elements radium and polonium, emitted similar radiation. What that radiation actually was, however, remained a mystery.
One interesting point about this important discovery was that it did not require new experimental apparatus or high technology. Becquerel used photographic plates, uranium salts, and other equipment already present in his laboratory. Crookes described Becquerel’s laboratory as follows: “What struck one as remarkable was the facility with which experimental apparatus was extemporized. Card, gummed paper, glass plates, sealing wax, copper wire, rapidly and almost spontaneously seemed to grow before one’s eyes into just the combination suitable to settle the point under investigation. The answer once obtained, the materials were put aside or modified so as to constitute a second interrogation of nature” (Crookes 1909, pp. xxi-xxii). Performing good experiments is an art.
1 J. J. Thomson and the Electron
The end of the nineteenth century and the beginning of the twentieth century were exciting and revolutionary times for physics. We have already noted the discovery of both x-rays and radioactivity. In 1900 Max Planck introduced quantization into physics, the idea that physical quantities can have only certain discrete values. In 1905 Albert Einstein introduced his special theory of relativity, which fundamentally changed our ideas of space and time. The revolutionary impact of these two theories is beyond the scope of this book, but they changed the way we think about nature and formed an integral and important part of the physics of the time.
In the midst of this exciting period, J. J. Thomson, and others provided evidence for a new elementary particle that was a fundamental constituent of atoms, the electron.* I will discuss Thomson’s work in some detail not only because the nature of the electron will be important in our discussion of β decay, but also because it presents an argument for the existence of a new elementary particle.
*. Although Thomson is usually, and with good reason, given credit for this discovery, the work of Wiechert, Kaufmann, and Zeeman all contributed to it.
The purpose of J. J. Thomson’s 1897 experiments was to investigate the nature of the then recently discovered cathode rays. He was attempting to decide between the view that the rays were negatively charged, material particles and the view that they were disturbances in the “aether,” the medium through which physicists at the time believed that light waves traveled. His first orde...
Table of contents
- Cover
- Half-Title
- Series
- Title
- Copyright
- Contents
- Preface to the First Edition
- Preface to the Second Edition
- Acknowledgements
- Chapter 1 The Road to the Neutrino
- Chapter 2 The Neutrino Hypothesis
- Chapter 3 Toward a Universal Fermi Interaction
- Chapter 4 Fermi’s Theory: The Final Act
- Chapter 5 “Observing” the Neutrino: The Reines-Cowan Experiments
- Chapter 6 How Much? The Mass of the Neutrino
- Chapter 7 How Many? Whose?
- Chapter 8 The Missing Solar Neutrinos
- Chapter 9 Neutrino Oscillations
- Chapter 10 Conclusion: There Are Neutrinos
- Epilogue: Neutrinos in the 21st Century
- References
- Index