
- 172 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
An attempt has been made to attract contributions which illustrate the importance of certain enzymatic processes involved in steroid biosynthesis and metabolism and, in some cases, leading to steroidal action in target sites. Investigators actively engaged in research in such areas were invited to present their material in a manner which they considered fitting. It is hoped that as a result if this, the publication will possess sufficient depth to warrant approval. The blend of review material and experimental data originating in the author laboratories will, it is felt, make for useful reading.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
STEROID Δ4-REDUCTASES: THEIR PHYSIOLOGICAL ROLE AND SIGNIFICANCE
TABLE OF CONTENTS
I. | Introduction |
II. | Corticoids |
III. | Aldosterone |
IV. | Progesterone |
V. | C19-Steroid Δ4-Reductases |
VI. | Summary |
References | |
I. INTRODUCTION
In this review of the steroid Δ4-reductases, the enzymes which catalyze the reduction of the double bond between carbons 4 and 5 of the steroid molecule, only mammalian steroids and mammalian enzymes will be discussed. The literature in this area of steroid metabolism is very voluminous and it will not be possible to quote all references on the topic. The general aim of the review is to indicate the physiological importance of Δ4-reduction in the overall disposition of steroid hormones and, in appropriate situations, how it affects biological actions of the parent compounds. A few general comments on the biochemistry of the enzymes will be made in this introductory section.
With the exception of the estrogens, which have an aromatic ring A, the other biologically important, naturally occurring mammalian steroid hormones have in general the Δ4-3-ketone structure in ring A. This group includes the important glucocorticoids cortisol and corticosterone, the mineralocorticoid aldosterone, the androgen testosterone, and the progestin progesterone. Key biosynthetic precursors of these hormones (such as l l-deoxycortisol, deoxycorticosterone, 18-hydroxycorticosterone, and Δ4-androstenedione) also have the Δ4-3-ketone configuration in ring A. Δ4-3-Keto-intermediates are involved in the conversion of cholesterol to bile acids.
The reduction of the double bond between carbons 4 and 5 of steroid molecules can either be the first step in a sequence of reactions leading to water-soluble conjugates which are excreted or result in the formation of compounds of key biological importance. The reduction of the double bond between steroid carbons 4 and 5 involves the addition of two hydrogen atoms. Because the reduction leads to the formation of an assyrnetric carbon atom at carbon 5, either of two possible isomers can result. By standard steroid nomenclature these are the 5α- (hydrogen below plane of steroid molecule) and the 5β- (hydrogen above plane of steroid molecule) reduction products (see Figure 1). Both isomers are formed in mammals.
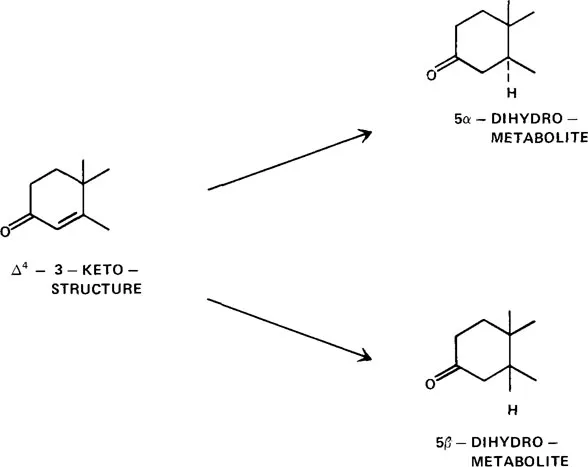
FIGURE 1. Steroid ring A reduction pathways yielding 5α- and 5β-metabolites.
As mentioned above, the enzymes catalyzing these reductions are called Δ4-reductases and can be either Δ4-5α-reductases or Δ4-5β-reductases, depending on the stereochemistry of the product at carbon 5. The Δ4-reductases catalyze an irreversible reduction as they are not capable of removing hydrogens from positions 4 and 5 of saturated steroids.
There have been two common approaches to the measurement of Δ4-reductase activity. Steroids with a conjugated double bond system in ring A (Δ4-3-ketone) have a characteristic absorption peak in the ultraviolet range in organic solvents at or close to 240 nm. This absorption peak disappears when the double bond between carbons 4 and 5 is reduced and, hence, this has provided the basis for many Δ4-reductase assays, particularly in the liver. The assay does not indicate in any way whether the product formed is a 5α- or a 5β-reduced steroid. However, in tissues other than liver, the rate of reduction is often not great enough to follow with changes in ultraviolet absorption spectra and 3H- or 14C-labeled steroids are utilized as substrates; the products are then isolated by standard chromatographic techniques and the rate of formation of the labeled products is used as the basis of assays for measuring Δ4-reductase activity.
The required cofactor for all these Δ4-reductases is NADPH. Bjorkhem1 examined the mechanism and stereochemistry of rat liver microsomal Δ4-5α-reductase, utilizing 7α-hydroxycholest-4-en-3-one as substrate, and found the hydrogen to be preferentially transferred from the 4B position of NADPH. The evidence obtained suggested a cis or nonspecific addition of hydrogen rather than a trans addition. Bjorkhem and Danielsson2 then investigated rat liver soluble Δ4-5β-reductase and found, using C27-, C24-, C21-, and C19--steroid substrates, that the 4A hydrogen of NADPH was transferred during the reduction reaction. Abul-Hajj3 confirmed the specificity of the 4B and 4A hydrogen transfer for the rat liver Δ4-5α-reductase and Δ4-5β-reductases, respectively, using testosterone as substrate.
When the details of Δ4-reductases for each of the hormone groups are examined, one notes that in general the liver contains both Δ4-5β-reductase and Δ4-5α-reductase activities while other tissues have only Δ4-5α-reductase activity. There are a few exceptions but in general there is no known biological significance to the existence of Δ4-5β-reductases is tissues other than the liver. For most steroids the liver is the principal site for their metabolism. There is considerable evidence that Δ4-reduction is the rate-limiting step in steroid metabolism and this is supported by the finding of low levels of 5α- and 5β-reduced 3-ketosteroids in the circulation. In other words, once the Δ4-reductases have performed their actions, other enzymes, in particular the 3-hydroxysteroid dehydrogenases, reduce the ring A saturated steroids to 3α- and 3β-hydroxysteroids, which in turn are then substrates for β-glucuronyl transferases and sulfokinases.
One other generalization for which examples will be noted later is that in the liver, Δ4-5β-reductases are soluble enzymes, i.e., they are found in the 105,000 × g cell homogenate supernatant, while the Δ4-5α-reductases are located in the microsomes or endoplasmic reticulum. In other tissues the Δ4-5α-reductases are also located in the microsomes and in the nuclei in some instances as well.
Details on the steroid Δ4-reductases will be discussed for the glucocorticoids (cortisol and corticosterone), aldosterone, progesterone, and C19-steroids. Those involved in bile acid metabolism will not be discussed. There have been studies on the formation of Δ4-reduced steroid metabolites in many species. Many in vivo studies have been conducted in humans, but these can be in some ways considered to be indirect in that the urine metabolites are used as indicators of the extent and type of Δ4-reduction. These studies are complemented in many instances by in vitro experiments with tissues, tissue fractions, and purified enzymes from animals. Such a situation is of course by necessity since the appropriate human tissues are often not readily available, especially in experimental situations where changes in enzyme levels are being followed. However, one must also remember the possibility of species differences when results for humans and animals are being compared. Because of the many studies in the literature. on the use of urine steroid metabolite levels to indicate or follow changes in steroid secretion by the adrenal cortex or gonads, examples of such will be given in greater detail for the corticoids since Δ4-reduction is not believed to be greatly involved in their actions. Similar principles apply to the androgens and progestins with regard to steroid metabolites and hence fewer details will be given for them. However, the involvement of Δ4-5α-reduction in the action of progestins and androgens will be detailed more for the latter two groups of steroids because its importance has been better documented; this applies in particular to the androgens.
II. CORTICOIDS
This section will deal with some of the products secreted by the adrenal cortex, in particular, glucocorticoids and mineralocorticoids. Not all related steroids will be mentioned as in some instances they are precursors of the biologically important compounds and they are all metabolized similarly in ring A. Cortisol, being the principal glucocorticoid in humans, has been the subject of a number of studies with regard to its metabolism. Until very recently no physiological significance has been attached to the Δ4-reduction of cortisol other than its role in the pathway leading to metabolites completely reduced in ring A which can be conjugated with glucosiduronic acid and excreted in the urine. Hence, the Δ4-reducion of cortisol is not involved directly in the expression of corticoid activity. The effects will only be indirect in that altered rates of liver Δ4-reduction can lead to changes in the amount of hormone available for the expression of activity. Recently, a case of hypertension with evidence of increased mineralocorticoid production has been reported in which the abnormality found in steroid metabolism was an increased excretion of unconjugated dihydrocortisol. This has stimulated some studies on the possible biological significance of such metabolites. There is also some indirect evidence for d...
Table of contents
- Cover
- Title Page
- Copyright Page
- Table of Contents
- Chapter 1 Steroid Δ4 Reductases: Their Physiological Role and Significance
- Chapter 2 Steroid Carboxylic Acids
- Chapter 3 Biochemistry of Steroid 17-Hydroxysteroid Dehydrogenases
- Chapter 4 The Role of Steroid Sulfatase and Sulfotransferase Enzymes in the Metabolism of C21 and C19 Steroids
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Steroid Biochemistry by R. Hobkirk in PDF and/or ePUB format, as well as other popular books in Medicine & Alternative & Complementary Medicine. We have over one million books available in our catalogue for you to explore.