
eBook - ePub
Fetal and Early Postnatal Programming and its Influence on Adult Health
This is a test
- 407 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fetal and Early Postnatal Programming and its Influence on Adult Health
Book details
Book preview
Table of contents
Citations
About This Book
There is a documented link between fetal nutrition and the development of disease risk in adult life. Including the early postnatal period, during which a newborn continues to grow rapidly influenced by environmental factors, suggests that individuals are subject to risks for more than just the fetal period. Fetal and Early Postnatal Programming and its Influence on Adult Health focuses on interrelated aspects of cellular programming related to early nutrition and this potential global health problem.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Fetal and Early Postnatal Programming and its Influence on Adult Health by Mulchand S. Patel,Jens H. Nielsen in PDF and/or ePUB format, as well as other popular books in Medicine & Nutrition, Dietics & Bariatrics. We have over one million books available in our catalogue for you to explore.
Information
Section IV
Human Studies on Early Programming
11 Vitamins and Programming of Noncommunicable Diseases
Chittaranjan Yajnik and Tejas Limaye
CONTENTS
11.1 Introduction
11.2 Physiological Role of Vitamins
11.3 One-Carbon Metabolic Pathways
11.4 Sources of Vitamins
11.5 Causes of Vitamin Deficiency in Pregnancy and Lactation
11.6 Maternal Vitamin Nutrition, Fetal Growth, and Development
11.7 Maternal Interventions and Offspring Health
11.8 Summary
Acknowledgment
References
11.1 INTRODUCTION
The conventional model of noncommunicable diseases (NCDs) envisages a genetic (and therefore nonmodifiable) susceptibility and precipitation by unfavorable lifestyle (unhealthy diet, physical inactivity, stress, and other). Therefore, prevention is usually equated with lifestyle change in adult life. Recent research has demonstrated an association between altered growth and development in early life and later susceptibility to NCDs.
The developmental origins of health and disease (DOHaD) hypothesis originated in the work of Prof. David Barker. DOHaD proposes that environmental factors that influence growth and development affect risk of NCD across the life course (Barker 2007). Maternal factors influencing intrauterine environment are of particular importance but postnatal factors affecting growth and development during infancy, childhood, and adolescence also contribute. A range of environmental factors are involved (Figure 11.1).
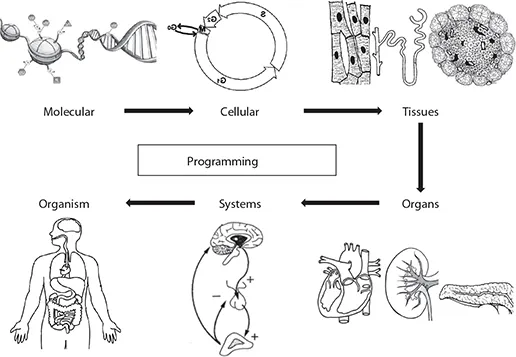
Environmental influences during critical stages of development permanently alter the structure and function of the cells, tissues, organs, and systems, which is called “programming” (Lucas 1991) (Figure 11.2). The idea originated in the demonstration of a link between small birth size and risk of chronic diseases in adulthood (Hales et al. 1991). Natural experiments of exposure to famine in utero are associated with a greater risk of chronic disease in later life (Roseboom et al. 2001). Fetal overnutrition in obese and diabetic pregnancies has a similar effect (Freinkel 1980; Dabelea and Pettitt 2001).
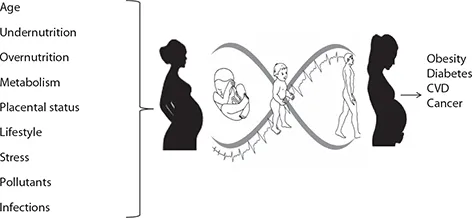
Maternal nutrition plays a critical role in fetal growth and development. The fetus depends on the mother for its nutritional needs, and, therefore, any disturbance in the maternal nutritional status or its supply to the fetus adversely impacts fetal growth. When nutrition is limited, it is preferentially used for the growth of the fetal brain by increased blood flow to the cephalic (preductal) system at the cost of caudal (post-ductal) and subdiaphragmatic organs like lungs, heart, liver, pancreas, and kidneys (Yajnik 2004). It makes the individual susceptible to a range of diseases, including sarcopenic adiposity, reduced β-cell mass, insulin resistance, and renal dysfunction. When challenged with adverse lifestyle these manifest as various NCDs.
Given the orchestrated nature of growth and development, there are periods (windows) when certain organs and systems are susceptible to environmental influences. Periconceptional, intrauterine, and postnatal periods may be the most influential in programming. The periconceptional period encompasses the natural “wiping out” of the inherited epigenome and the “reestablishment” of a new one (Morgan 2005). This period also covers vital processes, such as gametogenesis, fertilization, implantation, morphogenesis, embryogenesis, organogenesis, and placentation, all of which have a profound influence on the growth, development, differentiation, and phenotype of an individual (Steegers-Theunissen et al. 2013). A further window appears to be the adolescence when sexual development occurs. If the environment is not ideal (e.g., nutritional imbalance, altered metabolism, stress, infections, exposure to environmental pollutants), this might lead to undesirable programming and increase the risk for later disease (Figure 11.3).
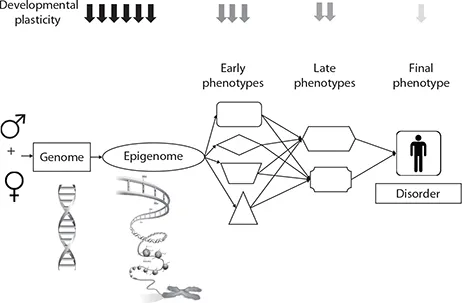
There is increasing recognition of an important role for micronutrients in these phenomena (Rao et al. 2001; Fall 2012). Micronutrients (vitamins and minerals) are essential for human health and development. It has been estimated that at least half of the children worldwide suffer from one or more micronutrient deficiency and globally more than two billion people are affected (Global Report 2009). Although micronutrient deficiencies during pregnancy have been associated with adverse pregnancy outcomes (Allen 2005), their effects on the long-term health of the offspring are only beginning to be understood. This chapter reviews some of the effects of maternal vitamin nutrition on the health of the offspring.
11.2 PHYSIOLOGICAL ROLE OF VITAMINS
Vitamins play an important role in the cellular function and metabolism and therefore affect practically all the processes in cells, tissues, organs, and systems. They act as antioxidants, coenzymes for many enzymes, and contribute to cell regulation, growth, and differentiation (Rolig 1986).
Vitamin A (retinol) is essential for normal vision, growth, reproduction, immunity, and epithelial tissue maintenance. Its influence is particularly critical during periods when cells proliferate rapidly and differentiate, such as during pregnancy and early childhood.
B complex vitamins affect the functions of various enzymes and influence a wide range of metabolic processes. This affects growth and development. Vitamins, which affect methyl group transfer, are particularly important (folate and vitamin B12, B6, B2) in modifying functions of various molecules (DNA, proteins, and lipids). This affects numerous biological processes like DNA biosynthesis, regulation of gene expression, chromatin structure, genomic repair, and stability.
Vitamin C (ascorbic acid) acts as a cofactor in a number of enzymatic reactions. Vitamin C is required for the synthesis of collagen, carnitine, catecholamine, and the neurotransmitter norepinephrine. It also acts as an antioxidant (protects against oxidative stress).
Vitamin D (cholecalciferol) regulates functions of various genes and influences cellular calcium metabolism. It plays a role in the function of the islet cells of pancreas, muscles, and immune system.
11.3 ONE-CARBON METABOLIC PATHWAYS
One-carbon (1-C) metabolism includes reactions, including the addition, transfer, or removal of 1-C units in cellular metabolic pathways (Kalhan and Marczewski 2012). The central methylation pathway, the methylation cycle (Figure 11.4), occurs in the cytoplasm of every cell where the S-adenosyl methionine (SAM) is generated from adenosine triphosphate (ATP) and methionine and is involved in a multitude of methylation reactions. In this process, SAM is converted to S-adenosyl homocysteine (SAH) and then to homocysteine. Methionine is regenerated when a methyl group from 5-CH3-tetrahydofolate is transferred to homocysteine by methionine synthase, which requires vitamin B12 as a cofactor. When concentrations of vitamin B12 are insufficient, folate becomes trapped as 5-methyl tetrahydrofolate, the regeneration of methionine is inhibited, and the concentrations of homocysteine are increased.
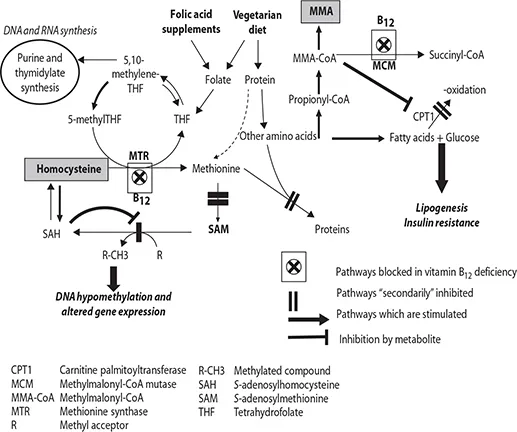
Raised concentrations of homocysteine within the cell are toxic and actively regulated by (1) remethylation, which requires vitamins B12 and B2, (2) transsulfuration, which requires vitamin B6, and (3) the transfer of one of the methyl groups of betaine to homocysteine to form methionine.
The remethylation of homocysteine to methionine is linked with the folate cycle, which generates methyl tetrahydrofolate, which is a cosubstrate for the methylation of homocysteine to methionine. The folate cycle is essential for purine and pyrimidine nucleotide synthesis, which is essential for the formation and stability of DNA, RNA, and nucleoside triphosphates such as ATP.
Methylation of DNA nucleotides is an important epigenetic mechanism for the control of gene expression. This control of gene expression is particularly important during critical periods of growth and development and may help explain why nutritional imbalances are associated with fetal phenotypes, which increase the risk for subsequent diseases (Rush et al. 2013; Yajnik and Deshmukh 2012).
In the mitochondria, β-oxidation of fatty acids requires that they are sequentially broken down into small even number carbon units, which enter the tricarboxylic acid (TCA) cycle for the ultimate oxidation of acetyl (two-carbon) groups derived from lipids. This generates carbon dioxide, water, and energy (Figure 11.4). Deficiency of vitamin B12 interferes with the transfer of long-chain fatty acyl-CoA into the mitochondria and inhibits β-oxidation, leading to accumulation of fatty acids in the cytosol and increased inclusion into glycerolipids. Thus, vitamin B12 influences folate-dependent reactions as well as mitochondrial energy generation.
11.4 SOURCES OF VITAMINS (FAO 2001; FIGURE 11.5)
Vitamin A is present in animal tissues, especially fish and liver. Provitamin A (carotenoids) is obtained from green leafy vegetables and red–orange colored fruits and vegetables and converted to vitamin A in the human body.
Folates are present in many natural foods, including dark-green vegetables, whole grains, and some nonvegetarian foods. It is also synthesized by intestinal bacteria. Vitamin B12 (cyanocobalamin) is synthesized exclusively by microorganisms and is obtained in the diet through consumption of animal source foods. Vitamin B12-containing plant-derived food sources include green and purple lavers (Nori) and blue–green algae/cyanobacteria (spirulina), but they may not suitable for humans due to the presence of biologically inactive pseudovitamin B12 (Watanabe 2007). Therefore, milk remains the only acceptable animal source of B12 for vegetarians.
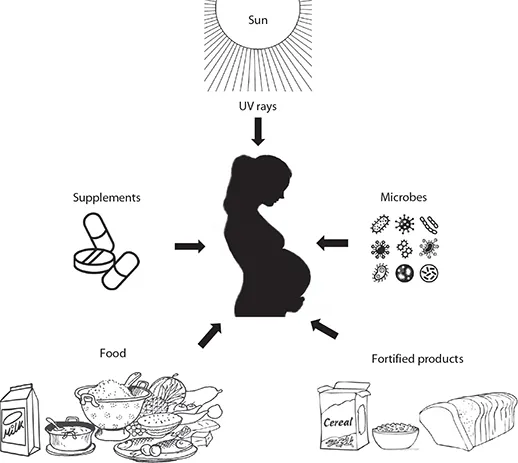
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Table of Contents
- Series Preface
- Preface
- Editors
- Contributors
- Section I: Development of the Early Programming Concept
- Section II: Maternal Malnutrition and Fetal Programming
- Section III: Early Postnatal Programming
- Section IV: Human Studies on Early Programming
- Section V: Epigenetic Mechanisms of Early Programming
- Section VI: Interventions
- Index