![]()
Section I
Synthetic Intermediates
![]()
11 Synthesis of 4-Nitrophenyl β-D-galactofuranoside
A Useful Substrate for β-D-Galactofuranosidases Studies
Carla Marino*, Santiago Poklepovich Caridea and Rosa M. de Lederkremer
Universidad de Buenos Aires, Pabellón II, Ciudad Universitaria
Sydney Villaume†
University of Namur (UNamur)
CONTENTS
Experimental
General Methods
4-Nitrophenyl 2,3,5,6-tetra-O-benzoyl-β-D-galactofuranoside
4-Nitrophenyl β-D-galactofuranoside
Acknowledgments
References
β-D-Galactofuranosyl units (β-D-Galf) are constituents of microorganisms, some of them pathogenic, such as Mycobacteria, the trypanosomatids Trypanosoma cruzi and Leishmania,1 and fungi such as Aspergillus fumigatus.2 Since Galf has never been found in mammals, its biosynthesis and metabolism are good targets for chemotherapeutic strategies. In some species, the degradation of Galf-containing glycoconjugates is promoted by extracellular β-D-galactofuranosidases. For example, Penicillium and Apergillius species,3 Helminthosporium sacchari,4 and Trichoderma harzianum5 produce exo β-D-galactofuranosidases (EC 3.2.1.146).
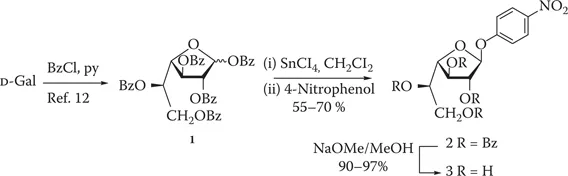
First studies of β-D-galactofuranosidases involved the use of methyl β-D-galactofuranoside as substrate and the tedious measurement of the reducing sugar released by the enzyme.4,6 The availability of the chromogenic substrate 4-nitrophenyl β-D-galactofuranoside (3), first described in our laboratory7 and later reported by Cousin et al.,8 significantly simplified this task. Since then, compound 3 has been extensively used for galactofuranosidase studies in P. fellutanum,9 and Aspergillius spp.8 Compound 3 has also been used as a substrate for chemoenzymatic syntheses.10,11 It used to be commercially available (Merck) but it was later discontinued. In our case, the starting compound was per-O-benzoyl-α,β-D-Galf (1),12 obtained in a single step from D-Gal, and the glycosylation was promoted by p-toluenesulfonic acid.7 The other laboratory started from per-O-acetyl-β-D-Galf, which required three steps for its synthesis, and the glycosylating agent was SnCl4,8 which proved to be very efficient for the synthesis of different galactofuranosides.13 In this chapter, we describe the synthesis of 3 by SnCl4-promoted glycosylation of easily available 1,12 followed by de-O-acylation.
EXPERIMENTAL
General Methods
Thin-layer chromatography (TLC) was performed on 0.2 mm silica gel 60 F254 (Merck) aluminum-supported plates. Detection was effected by UV light and by charring with 10% (v/v) H2SO4 in EtOH. Column chromatography was performed on silica gel 60 (230–400 mesh, Merck). The proton (1H) and carbon (13C) nuclear magnetic resonance (NMR) spectra were recorded with a Bruker AM 500 spectrometer at 500 MHz (1H) and 125.8 MHz (13C). Assignments were supported by homonuclear correlation spectroscopy (COSY) and heteronuclear single quantum coherence (HCQC) experiments. Optical rotations were measured with a Perkin-Elmer 343 polarimeter, with a path length of 1 dm. Melting points were determined with a Fisher-Johns apparatus and are uncorrected. CH2Cl2 was distilled from P2O5 and stored over 4 Å MS. MeOH was dried by refluxing over magnesium turnings and a little iodine, distilled, and stored over 4 Å MS.
Sodium methoxide was prepared by carefully reacting a sub-stoichiometric amount of sodium (∼0.020–0.025 g) with dried methanol (5 mL).
4-Nitrophenyl 2,3,5,6-tetra-O-benzoyl-β-D-galactofuranoside (2)
A 50 mL, single-neck round-bottom flask equipped with a rubber septum was charged with 1,2,3,5,6-penta-O-benzoyl-α,β-D-galactofuranose (1, 1.05 g, 1.50 mmol),* dry CH2Cl2 (10.0 mL), and 4 Å MS (0.1 g). The reaction vessel was flushed with argon and, with magnetic stirring and external cooling in an ice-water bath, SnCl4 (0.25 mL, 2.1 mmol, 99% purity)* was added, followed after 10 min by addition of 4-nitrophenol (0.25 g, 1.8 mmol)†. The cooling was removed, and the reaction mixture was allowed to reach room temperature.
After 3 h of stirring, TLC showed consumption of the starting material (Rf ∼0.6 both anomers, 9:1 toluene–EtOAc) and presence of main product (Rf 0.7). Excess 4-nitrophenol is also observed by TLC. The mixture was filtered, diluted with CH2Cl2 (50 mL), and successively washed with NaHCO3 (2.5%, 2 × 20 mL)‡ and sat aq NaCl (20 mL). The organic layer was dried (Na2SO4), filtered, and concentrated. The syrup obtained (1.95 g) was chromatographed (49:1 toluene–EtOAc) to afford syrupy 2 (654 mg, 61%), [α]D −11 (c 1, CHCl3).7 1H NMR (500 MHz, CDCl3) δ 8.16–7.15 (H-aromatic), 6.10 (m, 1 H, H-5), 6.07 (s, J < 0.5 Hz, 1 H, H-1), 5.78 (s, J < 0.5 Hz, 1 H, H-2), 5.77 (d, J 4.8 Hz, 1 H, H-3), 4.80 (apparent t, J 4.3 Hz, 1 H, H-4), 4.75 (m, 2 H, H-6, 6′); 13C NMR (50.3 MHz, CDCl3) δ 165.9, 165.7, 165.6, 165.4 (PhCO), 142.7–116.5 (C-aromatic), 103.8 (C-1), 83.1 (C-4), 81.9 (C-2), 77.3 (C-3), 69.9 (C-5), 63.0 (C-6). Anal. Calc. for C40H31NO12: C, 66.94; H, 4.35; N, 1.95. Found: C, 67.17; H, 4.36; N, 1.77.7
4-Nitrophenyl β-D-galactofuranoside (3)
A dry 100 mL, single-neck round-bottom flask with a magnetic stirring bar and a rubber septum was charged with 2 (0.5 g, 0.69 mmol) and dried in vacuo for 1 h at room temperature. Anhydrous CH2Cl2 (8 mL) was added and with stirring and external cooling in an ice-water bath 0.2 M methanolic NaOMe in anhydrous MeOH (2.5 mL) was added in one portion. After 30 min, TLC analysis (7:1:2 PrOH-28% aq NH3) showed complete conversion of the starting material into a more polar product (Rf 0.5), faster-moving than a galactose standard (Rf 0.2). The solution was concentrated to a small volu...