
eBook - ePub
Environmental Problems in Marine Biology
Methodological Aspects and Applications
This is a test
- 368 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Environmental Problems in Marine Biology
Methodological Aspects and Applications
Book details
Book preview
Table of contents
Citations
About This Book
Marine environment can be affected by several pollutants such as the presence of elements and their chemical species, pharmaceuticals, nanoparticles and other emerging contaminants. Environmental monitoring can be assessed by genomics, proteomics (i.e. redox proteomics), chemical speciation analysis and metallomics, metabolomics as well as other advanced strategies. The present book is a useful methodological tool for researchers and specialists in the field of analytical chemistry, environmental sciences, biochemistry, genomics and toxicology. The book includes for the first time the methodological aspects and applications related to chemical speciation and –omics strategies applied to marine environment.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Environmental Problems in Marine Biology by Tamara Garcia Barrera, Jose Luis Gomez Ariza in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
1
Element Speciation in Seawater
From Free Metal Determination to Proteomic Analysis
Department of Analytical Chemistry, Nutrition and Bromatology, Faculty of Chemistry, University of Santiago de Compostela 15782 Santiago de Compostela, Spain.
a Email: [email protected]
b Email: [email protected]
c Email: [email protected]
d Email: [email protected]
* Corresponding author
ABSTRACT
Various methods for trace element speciation in seawater samples are summarized in this chapter, ranging from free ions to metalloprotein analysis. These methods include separation and preconcentration techniques, such as SPE or HPLC, to avoid the problems due to the low concentration of the analytes and the interferences caused by the salinity of the samples. The combined use of both separation (HPLC, FFF, SDS-PAGE, or OFFGEL electrophoresis) and detection techniques (electrochemical methods, AAS, ICPMS, or ESI-MS) provides a wide number of applications to quantify and identify the different species in the marine environment.
Introduction
Trace elements are present in seawater originating from either natural or anthropogenic sources. The latter include chemicals used in the manufacture of pigments, paper, wood preservatives, pharmaceutical compounds, pesticides, etc. These trace elements enter the food chain, thus posing serious health hazards in some cases.
Trace elements can be classified as essential (Cu, Co, Fe, Mn, Mo, Zn, Cr, Ni, and Se) or toxic (Cd, Hg, Pb, Sb, Bi, Sn, and Tl) elements. The toxicity, mobility, and bioavailability of these elements depend on their concentration as well as their chemical form which determine their physical and chemical behavior in natural systems (Ure and Davison 2001). Trace elements can be present in natural aquatic systems in the form of free ions with different oxidation states and be associated to colloids and particles.
Speciation of Free Ions
Trace elements occur in seawater samples as free ions in different stages of oxidation. Some examples of species reported in the literature for elements such as antimony, arsenic, chromium, lead, mercury, selenium, tellurium, tin, and iodine are shown in Table 1. These species are present in seawater at very low concentrations usually at levels of few ng L−1 or μg L−1.
Table 1: Element species in seawater samples.
Element | Species |
Antimony | Inorganic antimony Sb(III) and Sb(V) |
Arsenic | arsenite (As(III)), arsenate (As(V)), monomethylarsonic (MMA), dimethylarsinic (DMA) |
Chromium | Cr(III), Cr(VI) |
Lead | Pb2+, triethyllead (TEL), trimethyllead (TML) |
Mercury | MeHg+, EtHg, and inorganic Hg |
Selenium | Se(IV), Se(VI) |
Tellurium | Te(IV) and Te(VI) |
Tin | MBT, DBT, TBT |
Iodine | Iodide, iodate |
The determination of these species in seawater samples presents some difficulties associated with their low concentrations and the salinity of the sample, which can produce interferences in analytical techniques. In order to solve these problems, highly sensitive analytical techniques are needed as well as preconcentration and separation steps to remove the matrix interferences.
Sample pretreatment
Several separation and preconcentration methods have been reported in the literature for element speciation in seawater. These methods include liquid-liquid extraction, solid phase extraction (SPE), solid phase microextraction (SPME), dispersive liquid-liquid microextraction (DLLME), preconcentration using knotted reactors, headspace-stir bar sorptive extraction, etc.
For SPE, a mini column filled with Amberlite XAD-8 resin has been used for Sb(III) preconcentration (Ozdemir et al. 2004). In this case, antimony was complexed with ammonium pyrrolidine dithiocarbamate (APDC) and quantitatively adsorbed in the column. The elution was performed using 10 mL of acetone. In a study by Calvo et al. (2011) [1,5-bis(2-pyrydil)-3-sulfophenylmethylene] thiocarbonohydrazyde immobilized on aminopropyl-controlled pore glass was used for Sb(III) preconcentration and Amberlite IRA-910 resin for Sb(V) preconcentration (Calvo et al. 2011). On-line preconcentration on an microcolumn packed with C18 bound silica gel has been reported in the literature for selective preconcentration of Cr(VI) as diethyldithiocarbamate complex (Prasada-Rao et al. 1998). An iminodiacetate resin (Muromac A-1) has also been used for Cr(III) separation and preconcentration (Hirati et al. 2000).
Solid phase extraction using ion-imprinted polymethacrylic microbeads has been used for mercury speciation before its determination by cold vapor atomic absorption spectrometry (Dakova et al. 2009). Metal ion-imprinted polymer particles were prepared by copolymerization of methacrylic acid as monomer, trimethylopropane trimethacrylate as cross-linking agent, and 2,2-azobisisobutyronitrile as initiator, in the presence of Hg(II)-1-(2-thizaolylazo)-2-naphthol complex. The adsorbed inorganic mercury was desorbed using 4 M HNO3. SPE using S. aureus loaded Dowex optipore V-493 columns has also been used for Hg speciation (Tuzen et al. 2009). Sequential elution with 0.1 M HCl and 2M HCl was performed after sample loading for MeHg+ and Hg2+, respectively.
Preconcentration of Hg(II) using poly(acrylamide) grafted onto cross-linked poly(4-vinyl pyridine) (P4-VP-g-PAm), in presence of MeHg+(I), has been investigated. The sorbent showed excellent selectivity for Hg(II) in presence of other ions such as, Pb(II), Zn(II), Cu(II), Cd(II) and Fe(III) (Yayayürük et al. 2011).
SPE has been used for Se(IV) separation and preconcentration (Saygi et al. 2007) in the form of Se(IV)-ammonium pyrrolidine dithiocarbamate chelate on Diaion HP-2MG. Total Se was determined after reduction of Se(VI) by heating the samples in a microwave oven with 4 M HCl.
Solid phase micro extraction (SPME) with fused silica fiber coated with polydimethylsiloxane (PDMS) has been used after derivatization of mercury species using sodium tetraphenylborate (Na(BPh4) (Carro et al. 2002; Bravo Sánchez et al. 2004; Mishra et al. 2005) allowing the determination of these species at very low concentrations (ng L−1). The only problem with this methodology is the blank contamination which can affect inorganic mercury evaluation. This problem was overcome by setting up cleaning procedures using minicolumns packed with 8-hydroxyquinoline to trap and remove mercury traces present in all the analytical reagents used (Bravo Sánchez et al. 2004). Head space SPME was further used for simultaneous determination of organometallic compounds of mercury, lead, and tin in seawater using a Divinyl benzene/carboxen/polydimethylsiloxane fiber (Beceiro et al. 2009). Selective magnetic SPE (MSPE) separation was used for speciation of inorganic tellurium from seawater (Huang and Hu 2008). In these case, Te(IV) was quantitatively adsorbed on γ-mercaptopropyltrimethoxysilane modified silica coated magnetic nanoparticles, within the pH range of 2–9, while Te(VI) was not retained thus remaining in the solution. The magnetic nanoparticles were then separated from the solution by applying an external magnetic field, and Te(IV), was recovered using a solution containing 2M HCl and K2Cr2O7.
Herbello et al. have used on-line sorption preconcentration in a knotted reactor for As(III) and Cr(VI) determination (Herbello et al. 2005, 2011). In this case, As(III) and Cr(VI) was complexed with ammonium pyrrolidine dithiocarbamate and adsorbed into the inner wall of the knotted reactor. The complexes were then eluted with ethanol (40 μL). Enrichment factors of 44 and 31 were obtained using this method for As(III) and Cr(VI) determination, respectively.
Organotin species have been extracted into toluene after addition of sodium ethyldithiocarbamate (DDTC) and NaCl to seawater samples (Tsunoi et al. 2000). Dispersive liquid-liquid microextraction (DLLME) and back extraction has been used for Hg2+ preconcentration (Li et al. 2011). Mercury was extracted with 1-(2-pyridylazo)-2-naphthol (PAN) in ethanol and back extraction was performed with L-cysteine. This method resulted in an enrichment factor of 625.
Analytical techniques
Several analytical techniques have been used for the determination of trace element species in seawater samples, including electrochemical methods, catalytic spectrophotometry, atomic absorption spectrometry (AAS), atomic fluorescence spectroscopy (AFS), inductively coupled plasma atomic emission spectroscopy (ICP-AES), inductively coupled plasma mass spectrometry and high performance liquid chromatography (HPLC), capillary electrophoresis (CE), and gas chromatography (GC) coupled with various detectors.
Electrochemical methods present certain advantages over other methods for element speciation in seawater, including high sensitivity and selectivity associated with low detection thresholds. The seawater matrix is an “ideal” electrolyte to perform the measurements with minimal sample preparation, low-cost of analysis, and requiring minimal analysis times (Riso et al. 2004). Electrochemical methods used for trace speciation analysis are based on differential pulse anodic/cathodic stripping voltammetry (Quentel and Filella 2002; Papoff et al. 1998; Quentel and Elleouet 1999; Nascimiento et al. 2009), anodic stripping voltammetry with a gold microelectrode (Salaün et al. 2007), adsorptive cathodic stripping voltammetry (AdCSV) (Carballo et al. 2008), stripping chronopotentiometry (SPC) (Riso et al. 2004), and stripping chronopotentiometry with a mercury film electrode (Tanguy et al. 2010). Limits of detection (LOD) in within the range of a few ng L−1 were obtained using these techniques. As an example, LOD of 8 ng L−1 was obtained for Sb(III) determination using stripping chronopotentiometry with a mercury film electrode (Tanguy et al. 2010), and an LOD of 0.16 ng L−1 for Se(IV) using differential pulse cathodic stripping voltammetry (Papoff et al. 1998).
Catalytic spectrophotometry is an analytical technique used for iodine speciation (Truesdale et al. 2003a; Truesdale et al. 2003b). Total iodine is determined by catalytic spectrophotometry (arsenious acid plus Ce(IV) sulphate reaction catalyzed by iodide at 34°C). Iodate is determined by spectrophotometry (conversion of iodate to I3− ion with potassium iodide in sulphamic acid).
Atomic absorption spectroscopy techniques including flame atomic absorption spectroscopy (FAAS), electrothermal atomic absorption spectroscopy (ETAAS) and cold vapor atomic absorption spectroscopy (CVAAS), Hydride Generation Atomic Absorption Spectroscopy (HGAAS), have also been used in speciation studies. In these cases, preconcentration and separation steps are needed before the determination. This can be performed off-line or on-line using solid phase extraction procedures, with different sorbents (ion-imprinted polymethacrylic microbeads, C18 bounded silica gel, etc.), coprecipitation, or using knotted reactors. Some methods reported in the literature for the determination of As, Cr, Hg, Se, Sb, and Sn using AAS, are summarized in Table 2. This table also shows the sample preparation step and the LODs obtained with these methods.
Atomic fluorescence spectroscopy (AFS) is a highly sensitive technique also used in speciation studies. Limits of detection of 13 and 15 ng L−1 were obtained for As(III) and As(V), respectively, using this technique. The method was based on the generation of arsine (AsH3) from the reaction between the arsenic species in the injected solution and tetrahydroborate immobilized on a strong anion-exchange resin (Amberlite IRA-400). Speciation was based on two different measurement conditions: (i) acidification to 0.7 M with HCl, (ii) acidification to 0.1 M with HCl in the presence of 0.5% L-cysteine, resulting in two calibration equations with different sensitivities for each species (Wang and Tyson 2014).
Cold vapor atomic absorption spectroscopy has been used for Hg2+ and MeHg+. In this case, mercury species were preconcentrated using poly(acrilamide) grafted onto cross-linked poly(4-vinyl pyridine) and eluted using 2 mL of HNO3 (14.3 M). An LOD of 2 ng L−1 was obtained (Yayayürük et al. 2011).
Chromatographic techniques have been widely used in speciation studies coupled with different detectors, such as AFS, ICP-AES, ICP-MS, MS, MS/MS. HPLC coupled to ICP-MS offered certain advantages in these studies due to their possibility of multielemental determination, as well as high sensitivity. Some applications using HPLC coupled with different detectors are summarized in Table 3. Gas chromatography has a large number of applications for lead, mercury, and tin speciation in seawater samples. A derivatization step, using sodium tetra phenyl borate or sodium tetra ethyl borate, is usually included before the chromatographic separation. Some applications reported in the literature using gas chromatography for trace element speciation in seawater samples are summarized in Table 4. As can be observed in Tables 3 and 4, LODs of a few ng L−1 are reported using these techniques.
Capillary Zone Electrophoresis (CZE) with diode array detection (DAD) has been used for Hg2+ determination in seawater samples. Hg2+ was preconcentrated by dispersive liquid-liquid microextraction-back extraction (DLLME-BE) using 1-(2-Pyridylazo)-2-naphthol (PAN) and L-cysteine as chelating reagents for DLLME and BE, respectively. An LOD of 0.62 μg L−1 has been obtained (Li et al. 2011). CE-UV has been used for direct analysis of iodine species in seawater (Huang et al. 2004a,b), obtaining LODs of 0.23 and 10 μg L−1 for iodide and iodate, respectively.
Complexes of Trace Metals with Organic Ligands
Dissolved organic matter (DOM) plays a crucial role in the transport and fate of nutrients and trace micropollutants (trace metals included) in surface waters and also in seawater (Ogawa and Tanoue 2003; Park 2009). The presence of several oxygen, nitrogen, and sulfur-containing functional groups such as carboxylic, phenolic, alcohol, amino acid, and thiol, is responsible for the DOM complexation properties (Wu et al. 2004).
Trace elements can exist as different species in natural waters: free aquatic ionic species, dissolved inorganic or organic complexes, complexes with colloidal or particulate matter (inorganic or organic), and complexes associated with the biota. Labile metal fractions are usually the most toxic, with some exceptions, such as Hg or Sn. The labile fractions of trace metals determined by ion exchange methods are generally greater than those obtained by voltammetric techniques, owing to the longer contact times in ion exchange preconcentration (Jiann and Presley 2002).
Table 2: Speciation studies using Atomic Absorption Spectrometry.
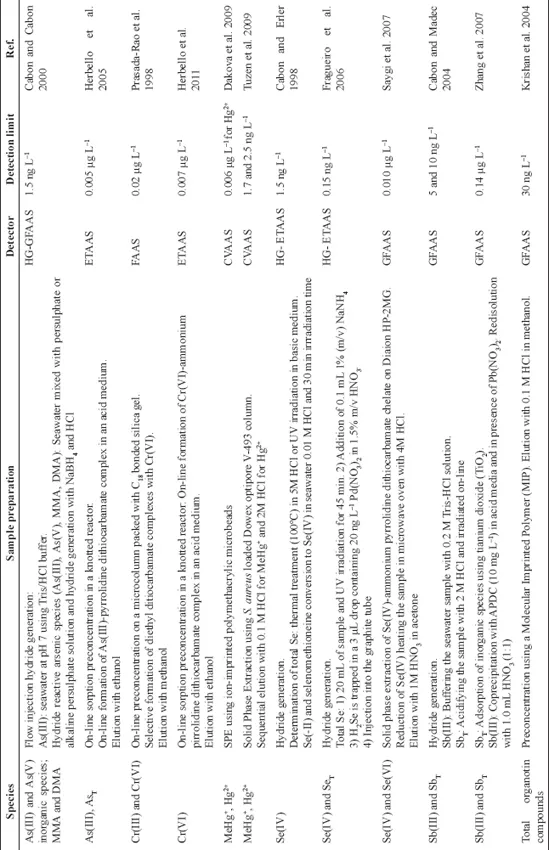
Table 3: Analytical methods for trace element speciation using HPLC.
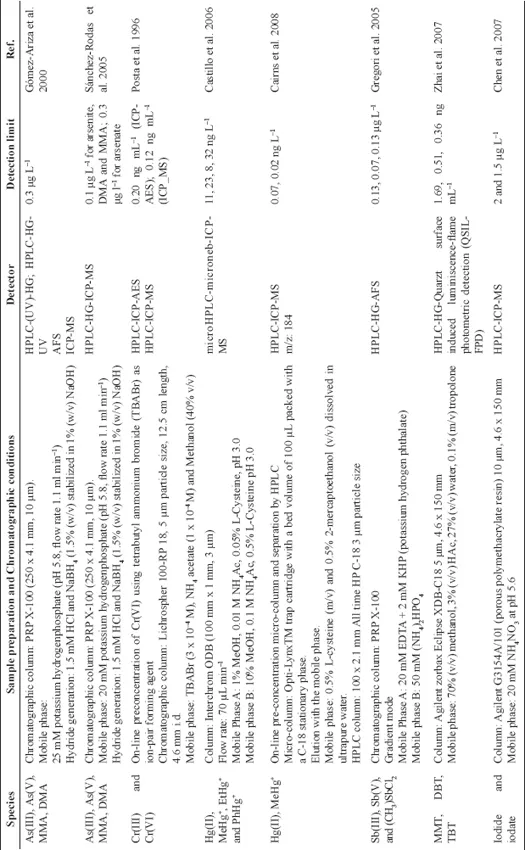
Table 4: Analytical metho...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Abbreviations
- 1. Element Speciation in Seawater: From Free Metal Determination to Proteomic Analysis
- 2. The Decisive Role of ICPMS and Related Hyphenated Techniques in Multielement and Speciation Analysis of Marine Biota
- 3. Mercury Speciation in Marine Biota and its Influence on Human Health: Metallomic and Metabolomic Approaches
- 4. Selenium Speciation in Marine Environment
- 5. Arsenic Occurrence in Marine Biota: The Analytical Approach
- 6. Biological Effects of Organotins in the Marine Environment
- 7. Functional Genomics Approaches in Marine Pollution and Aquaculture
- 8. New Trends in Aquatic Pollution Monitoring: From Conventional Biomarkers to Environmental Proteomics
- 9. Protein Expression Profiles in Marine Organisms Exposed to Nanoparticles
- 10. Effects of Climate Change on Marine Organisms: A Proteomic Approach
- 11. Environmental Metabolomics and Toxicometabolomics in Marine Pollution Assessment
- 12. Fate and Toxicity of Inorganic Engineered Nanomaterials in the Marine Environment: Analytical Techniques and Methods
- 13. Pharmaceuticals in the Marine Environment: Analytical Techniques and Applications
- 14. Biological Effects of Pharmaceuticals in Marine Environment
- Index