
eBook - ePub
Telomeres, Diet and Human Disease
Advances and Therapeutic Opportunities
This is a test
- 178 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Telomeres, Diet and Human Disease
Advances and Therapeutic Opportunities
Book details
Book preview
Table of contents
Citations
About This Book
The maintenance of telomeres—repetitive sequences at the end of chromosome—is essential to health. Dysfunction in telomere maintenance pathways plays a role in aging, cancer, atherosclerosis and other diseases. This has led to telomere maintenance as a prime target for patient therapies. This book describes the advances in telomere research as it applies to human health and especially how lifestyle and dietary factors could modify the telomerase maintenance process. The book examines the mechanisms involved, the primary of which are oxidative stress and the role of sirtuins, and how they can be modified by dietary patterns such as Mediterranean diet.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Telomeres, Diet and Human Disease by Amelia Marti, Guillermo Zalba, Amelia Marti, Guillermo Zalba in PDF and/or ePUB format, as well as other popular books in Medizin & Ernährung, Diätetik & Bariatrie. We have over one million books available in our catalogue for you to explore.
Information
Contents
Preface
1. Homeostasis of DNA Integrity
Marcela Segatto and Maria Isabel Nogueira Cano
2. Telomerase Activity
Daniel Eduardo Gómez, Diego Luis Mengual Gómez, Lina Maloukh and Romina Gabriela Armando
3. Measurement of Telomere Length
Álvaro Pejenaute and Guillermo Zalba Goñi
4. Telomere Integrity and Length and Cancer Risk
Jian Gu and Xifeng Wu
5. Telomere Length in Cardiovascular Disease
Marilena Giannoudi and Ioakim Spyridopoulos
6. Environmental and Occupational Exposure and Telomere Length
Natalia Pawlas
7. Telomeres and Physical Activity
Jean Woo, Ruby Yu and Nelson Tang
8. Caloric Restriction, Sirtuins, and Ageing
Angelo Avogaro and Saula Vigili de Kreutzenberg
9. Role of Dietary Pattern and Obesity on Telomere Homeostasis
Sonia García-Calzón and Amelia Marti del Moral
10. Advanced Research in Telomeres and Disease Risk
Virginia Boccardi, Irene Tassi and Patrizia Mecocci
11. Clinical Aspects Relevant to Telomere Maintenance and Therapeutic Opportunities
Brooke R. Druliner, Mohamad Mouchli and Lisa A. Boardman
Index
Preface
This volume includes 11 contributions from an international panel of experts which cover from basic science to clinical relevant work concerning Human Telomere Homeostasis.
The major aim of the present book is to provide an in depth review of our current knowledge on human telomere integrity in regard to the risk of major diseases and also examine the influence of lifestyle choices. It also illustrates future directions in research on ageing, nutrition, telomeres, and relevant diseases such as cancer, and cardiovascular diseases.
The first part of the volume focuses on general aspects of telomere biology, mainly the molecular mechanisms underlying the telomere machinery, the role of telomerase activity and the current methods to measure telomere length.
A review of the evidence on Telomere length, aging and disease risk is reported in the second part. Since telomere length is considered as a marker of cellular and biological aging, several epidemiological studies have investigated the association between telomere length and aging-related diseases.
The third part is devoted to the influence of lifestyle choice on telomere integrity. It is well known that, environmental factors can modify telomere length and this area is receiving major attention in disease prevention and treatment. Successful lifestyle interventions could lessen telomere attrition, like those based on Mediterranean diet.
The fourth part deals with clinical aspect relevant to telomere maintenance and therapeutic opportunities. Chronic oxidative stress, together with a reduction in anti- ageing molecules (sirtuins), accelerates the ageing process and is involved in the pathophysiology of chronic diseases. Understanding these molecular mechanisms has provided evidence for novel therapeutic targets that may slow the ageing process. Dysfunction in telomere maintenance pathways plays a role in aging, cancer, atherosclerosis and other diseases. This has led to telomere maintenance as a prime target for patient therapies.
As demonstrated in this volume, the inter-disciplinary approach has yielded a rich harvest of basic knowledge concerning telomere integrity and will provide the seeds for future breakthroughs in clinical progress. The text is intended to furnish the reader with a general view of the state of the art in this novel area of research. We will be satisfied if the multidisciplinary nature of these proceedings informs and stimulates the readers. It is our hope that the volume will encourage further research and understanding of all aspects of this intriguing and complex telomere field.
The editors express their gratitude to the authors of the articles and to CRC Press for having made possible the publication of this volume.
Amelia Marti del Moral
Guillermo Zalba Goñi
Guillermo Zalba Goñi
Chapter 1
Homeostasis of DNA Integrity
INTRODUCTION
Homeostasis comprises the tendency of an organism or cell to regulate their chemical processes that take place internally so as to maintain health, vital functions and stability, owing to the coordinated response of its parts to any situation or stimulus tending to disturb its normal condition or function. But how do cells work in order to preserve DNA homeostasis? The diverse stimuli that affect DNA integrity and stability, such as changes in the genome and in gene expression can disrupt the stable state of the cell with repercussions in pathways that regulate apoptosis, senescence, and cancer. In this chapter, we review the principal mechanisms involved in DNA homeostasis, pointing our interest in the role that telomere structure and its regulation play in this field.1, 2, *
Cellular Homeostasis and the Damaging Factors that Interfere with DNA Integrity and Stability
Because DNA is the repository of genetic information in each living cell, maintaining its integrity and stability is essential to life. Over their lives, organisms are exposed to many different environmental and internal stimuli that affect or modify their functionality. In fact, it has been estimated that one genome can suffer up to ten of thousands of changes per day (Lindahl and Nyberg 1972). Cells reply to DNA damage by activating complex signaling networks that decide cell destiny, supporting not only DNA repair and survival but also cell death. This decision depends on factors that are involved in DNA injure recognition, DNA repair and damage tolerance, as well as on elements involved in the activation of apoptosis, necrosis, autophagy and senescence (Roos et al. 2016) (Fig. 1).
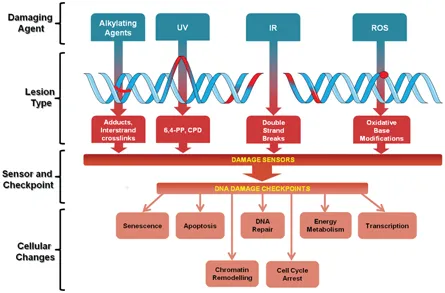
Fig. 1 DNA damage & repair: mechanisms for maintaining DNA homeostasis or induction of cell death. The genome is under constant threat since it is regularly exposed to endogenous and exogenous agents that may damage DNA. Cells have a repertoire to sense and activate DNA damage checkpoint proteins in face of different type of lesions. Ultimately, the consequences of DNA damage are activation of DNA repair pathways, of chromatin remodeling factors, modifications of transcription, fine tuning of energy metabolism, cell cycle arrest, and in case of irreparable damage, induction of senescence or apoptosis. (UV) ultraviolet radiation contained in sunlight; (IR) ionizing radiation due to, e.g., cancer therapy; (ROS) reactive oxygen species; (CPDs) cyclobutane pyrimidine dimmers; (6,4-PP) 6,4-photoproducts. (Adapted from Wolters and Schumacher 2013).
The genome is under constant threat since it is regularly exposed to exogenous agents that damage DNA. Prolonged exposure to pollutants, metals, toxic compounds, ultraviolet (UV) and ionizing radiation are some examples. Ionizing radiation typically leads to single and double-strand breaks. One example of the link between environmental-induced DNA damage and disease is that of skin cancer, caused by excessive exposure to sunlight UV radiation, which triggers two classes of DNA lesions: pyrimidine dimers and 6-4 photoproducts (Hurley 2002). These lesions distort DNA’s structure impeding transcription and replication. One “hot spot” for UV-induced injure is found within the frequently mutated-tumor suppressor p53 gene, considered the “genome guardian” (Clancy 2008).
Endogenous processes can also induce DNA damage. Some examples are hydrolysis that leads to spontaneous DNA depurination, DNA alkylation can lead to adduct and interstrand crosslink formation, reactive oxygen species (ROS) induce base oxidation and DNA breaks and replication fork collapse that can result in strand breaks (Sancar et al. 2004). These DNA lesions must be repaired to prevent loss or incorrect transmission of genetic information because errors can cause abnormal development and tumorigenesis. However, which repair system to use depends on the type of lesion, type of cell and on the cell-cycle phase (Branzei 2008). The way cells do to restore DNA integrity will be discussed in the next section. Before we review how cells usually keep their genetic integrity in the replication context.
DNA Replication—General Aspects
DNA replication is a process in which all organisms must duplicate their genome with extreme accuracy before each cell division. Replication occurs in four main steps: recognition of origins of replication by different factors and assembling of the pre- replication complex in the beginning of each cell cycle will license the replication initiation at S phase, followed by the opening of the double helix and separation of the DNA strands, the priming of the template strand, and the assembly of the new DNA segment. The two strands of the double helix DNA uncoil at a specific location, known as Origin of Replication, where several enzymes and proteins will work together to prepare the strands for semi-conservative replication. Finally, special enzymes called DNA polymerases organize the assembly of the new DNA strands. This four-stage process generally applies to all cells, but specific variations may occur (Alberts 2014).
After cells divide and at the beginning of G1 phase, the pre-replication complex including the DNA helicase complex (MCM 2-7) assembles onto the DNA throughout the G1 phase (Blow and Dutta 2005). At the end of G1, cell-cycle dependent kinases trigger the initiation of DNA replication to origins of replication, which in eukaryotes are scattered throughout the genome. How cells control which origin will fire is not exactly known, because not necessarily one origin is activated every cell cycle, most of them remain dormant (McIntosh and Blow 2012). But an input from the cell cycle machinery is activated in a way that each active origin can fire only once per cell cycle and the space between them must guarantee that the whole genome is replicated during the S phase. DNA replication takes place at a Y-shaped structure named replication fork. A DNA polymerase enzyme catalyzes nucleotide p...
Table of contents
- Cover
- Halftitle
- Title
- Copyright
- Table of Contents